Citric Acid Formula
Citric acid is an organic chemical compound which is found in citric fruits. It is largely used by the food industry as acidifier and flavoring.
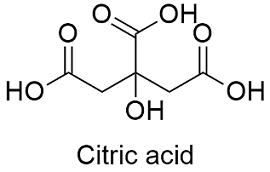
Formula and structure: The citric acid chemical formula is C6H8O7 and its extended formula is CH2COOH-C(OH)COOH-CH2 COOH. Its molar mass is 192.12 g mol-1. The molecule is formed by three carboxylic acid groups and a hydroxyl group. The four groups are linked to a 5 carbons chain. The molecule has a planar structure in the carboxylic group, however the main chain also has 3 tetrahedral carbons. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Citric acid is an organic chemical compound which is found in citric fruits and vegetables. It has a very important function on the citric acid cycle, which occurs in aerobic organisms.
Preparation: Citric acid is prepared from the juice of some fruits such as lemons and oranges. The acid is extracted from these juices or also from pineapple juice. However, the most extended method is the fermentation of sugar cane or corn syrup by Aspergillus or other fungi genus to obtain citric acid.
Physical properties: Citric acid is a odorless, crystalline white solid. Its density is 1.665 (the anhydrous form) and 1.542 (monohydrate) g mL-1 and the melting and boiling points are 156 ºC and 310 ºC. It is very soluble in water, ether, acetone, ethanol and methanol and it is insoluble in toluene, benzene and dichloromethane.
Chemical properties: Citric acid is a weak acid, thus in aqueous solution it is always in equilibrium between the anionic -1, -2 and -3 form and the neutral form. The most negative charge the anion has, the most acidic it will be, varying the pH of the corresponding aquous solution between pH 2 (for the CH2COOH-C(OH)COOH-CH2COO-) and pH 8 (CH2 COO-C(OH)COO-CH2COO-3). For this reason, citric acid is used as a buffer regulator of pH in many industrial processes. Other important characteristic of the citric acid is its capacity to chelate metals forming chelate metals similar to the EDTA complexes.
Uses: Citric acid is mostly used as acidifier on industrial processes. It is highly demanded to regulate pH in food and beverage preparation and preservation. It is also applied on the cosmetic and pharmaceutical manufacture. Moreover, citric acid is essential in some metabolic pathways of plants and bacterial, through a cycle known as citric acid cycle or Krebs cycle.
Health effects / safety hazards: Citric acid can b toxic when inhaled or ingested in large quantities. It can also irritate eyes and mucous. Citric acid is not flammable but it is corrosive, also to skin and some metals.
|
Related Links: |
Related Topics
Citric acid Formula
Acid Names Formulas
Chemistry Formulas
Formulas: Physics Formulas and Math Formulas
General Chemistry topics
Normality Formula
