Citric acid Formula
Citric acid is an organic tricarboxylic acid which is an important metabolite in all animals and plants.
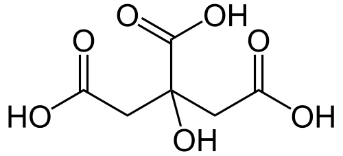
Formula and structure: The molecular formula of citric acid is C6H8O7 and its molar mass is 192.12 g/mol. The chemical structure of citric acid is shown below. Citric acid is an alpha-hydroxy acid with a three carbon skeleton, which has three carboxylic acid groups (COOH), and one hydroxyl group (OH).
Occurrence: Citric acid occurs naturally in many fruits and vegetables, with the largest amounts in citrus fruits such as oranges, lemons and limes. It is also an important metabolic intermediate in the biochemical citric acid cycle and is present in all living things.
Preparation: Citric acid is mainly produced by the microbial fermentation of carbohydrates such as molasses, corn sugar, cane sugar, beet, etc. It is also extracted from citrus fruits as they contain up to 8% of citric acid.
Physical properties: Citric acid is found as odorless and colorless crystals with an acidic taste. The solid has density of 1.66 g/mL, melting point of 153 °C and boiling point of 175 °C. It is highly soluble in water to give an acidic, sour tasting solution.
Chemical properties: Citric acid is a weak organic acid. It is a tribasic acid, as it has three COOH groups that can react with three base molecules. It commonly exists as anhydrous (water-free) form or as a monohydrate (with one molecule of water). The monohydrate can be converted to the anhydrous form when it is heated to about 78 °C. When heated to temperatures above 175 °C, it decomposes with loss of carbon dioxide. Citric acid readily forms citrate complexes with metallic cations.
Uses: Citric acid has many uses in the food industry as a flavoring agent, pH modifier, and preservative. It is also used as an anticoagulant and antioxidant, and in mineral supplements as citrate salts of metals. It also finds uses in household cleaning solutions, as a pH buffer and as a water softener.
Health effects/safety hazards: Dilute solutions of citric acid are safe for consumption. However, concentrated solutions or pure citric acid can be irritating and corrosive, and burn eyes and skin upon contact. Inhalation can irritate the nose, throat, and mucous membranes.
|
Related Links: |
Related Topics
Acid Names Formulas
Chemistry Formulas
Formulas: Physics Formulas and Math Formulas
General Chemistry topics
Normality Formula
