Chlorous Acid Formula
Chlorous acid, also known as chlorige Saeure, is an inorganic compound used in the production of chloride dioxide.
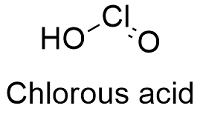
Formula and structure: Chlorous acid chemical formula is HClO2 and the molar mass is 68.46 g mol-1. The structure is formed by one proton H+, which is attached to a chlorite anion ClO2-. The extended formula is O=Cl-OH. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Chlorous acid is extremely unstable, so that it cannot be found in nature as a free compound.
Preparation: Chlorous acid can be prepared from the reaction of barium chlorite with sulfuric acid:
Ba(ClO2)2 + H2SO4 → BaSO4 + 2 HClO2
It can also be synthesized using lead chlorite instead of barium chlorite:
Pb(ClO2)2 + H2SO4 → PbSO4 + 2 HClO2
Physical properties: The physical properties of chlorous acid have not been correctly determined due to its high instability. It has a pKa 1.96 pKa is determined as the stability of the conjugated base of a determined acid. A value of 1.96 means the conjugated base chlorite ClO2- is stable.
Chemical properties: Chlorous acid is unstable and it reacts to form hypochlorous acid and chloric acid, one of them comes from the reduction of Cl3+ to Cl+1 and the second from the oxidation of Cl3+ to Cl5+:
2 HClO2 → HClO + HClO3
Uses: The use of chlorous acid is very limited due to its instability. However, its conjugated base chlorite is used as a salt of sodium that is used in some industrial processes as the production of chlorine dioxide.
Health effects / safety hazards: As many compounds that contains chlorine, chlorous acid is extremely harmful if swallowed or inhaled. It can cause severe damages in eyes, mucous and skin. It is a strong oxidizing agent; however its reactivity is limited by its poor stability.
|
Related Links: |
