Chlorite Formula
Chlorite ion, also known as chlorine dioxide ion, is chemical specie formed of oxygen and chlorine which is the anion of many chlorite salts used, for example, in disinfection.
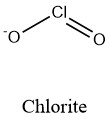
Formula and structure: The chlorite ion chemical formula is ClO2-. The molar mass is 67.45 g/mol. The ion comes from the deprotonation of the chlorous acid HClO2. The structure is formed by a chlorous centered cation Cl+3 and two oxygen anions O2- joined through a double and a single ionic bond respectively. The ion has a total charge of -1, which makes it an anion that can react with cations to form chlorite salts. The geometry of the molecule is a bent geometry, similar to a V with an angle of 111° between the Cl-O-Cl. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Chlorite in is not found in nature.
Preparation: Chlorite ion is prepared from its corresponding acid, the HClO2. It only exists in aqueous solution and is has not been prepared in other forms.
Physical properties: Chlorite ion as mentioned before is only found in aqueous solution. As all the ions, it does not have specific physical properties and the properties depends on the cation to which is joined to form the chlorite salts. For example, sodium chlorite NaClO2 is a white to yellow solid stable solid while silver chloride is explosive and instable.
Chemical properties: Sodium chlorite is a one of the diverse chlorite salts that are formed with the combination of the clorine-oxygen anion and diverse cations. The diversity of it is due to the number of oxidation states of the chlorine: Cl-, Cl+, Cl3+, Cl5+, or Cl7+. In the case of the sodium salts, it would be formed: NaCl (sodium chlorite), NaClO (sodium hypochlorite), NaClO2 (sodium chlorite), NaClO3 (sodium chlorate), NaClO4 (sodium perchlorate).
Uses: The most common chlorite salt, sodium chlorite is used for disinfection processes where it acts as germicide. It can be used in treatment of waters for humans’ consumption. Sodium chlorite is also used as a blenching agent in textiles industry. It is also used in blenching of papers.
Health effects / safety hazards: Sodium chlorite is not dangerous or toxic in low concentrations. However it can be poisonous if concentrated. It is not flammable. Other chlorites are toxic for human and the environment.
|
Related Links: |
