Chlorine Gas Formula
Chlorine gas is an inorganic gas, polemic for being used as chemical weapons, it is mostly used in controlled quantities in chemical industry.
Formula and structure: the chemical structure of chlorine gas is Cl2 and its molecular weight is 70 g/mol. Its structure is Cl-Cl, which is also called the element form of the chlorine element, consist in 2 atoms of chlorine joined by a covalent bond. Both atoms, have a sp3 configuration, i.e, their structur is tetrahedrical. Its chemical structure can be written as below, in the common representations used for organic molecules.
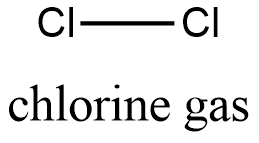
Occurrence: Chlorine gas is extremely reactive for being found in nature, however, the chlorine ion is largely found in nature, especially as the form of inorganic salts as NaCl.
Preparation: Chlorine gas is produced through several methods such as the chlor-alkali, in which a solution of NaCl is decomposed electrolytically by application of a current, the method produce hydrogen gas, chlorine gas and sodium hydroxide.
2 NaCl + 2 H2O → Cl2 + H2 + 2 NaOH
This process is cheap due to the sodium chlorine is extracted from seawater, lakes or mineral deposits.
A second method is the preparation of chlorine by combining hydrochloric acid and manganese dioxide.
Physical properties: Chlorine is a greenish yellow with a pungent suffocating odor gas. It liquefies at 35 ºC and it is slightly soluble in water. It is sold as liquefied compressed gas.
Chemical properties: Chlorine gas shows many of the characteristics of elementary chlorine. It is the elementary form of the chlorine element, due to the configuration of the Chlorine atom is [Ne]3s23p5, being to instable for existing as isolated specie, however, when two atom of chlorine form a bond, they share one electron, making with both of them get the electronic configuration [Ar], that is much stable.
Uses: Chlorine gas is used in the treatment of water due to its disinfectant properties. It is used by the paper industries as a bleaching agent.
Health effects/safety hazards: when concentrate, vinegar is very harmful, being fatal when inhaled. It causes serious eye irritation and skin irritation. It can also case fire due to it is a strong oxidized agent. It is toxic to aquatic life.
|
Related Links: |
