Carbon monoxide Formula
Carbon monoxide, also known as carbonous oxide or just monoxide, is a poisonous gas known for causing nervous system damage and asphyxiation. It is used in some chemical processes to produce methanol and acrylates.
Formula and structure: The carbon monoxide chemical formula is CO. The molar mass is 28.01 g/mol. The molecule is formed one carbon atom and one oxygen atom joined by one triple bond. As in all triple bonds, it is formed by one sigma bond and two pi bonds and the molecule acquire a linear geometry. Its chemical structure can be written as below, in the common representations used for organic molecules.
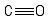
Occurrence: Carbon monoxide is found in nature, as produced in low concentrations by some archaea and bacteria. Carbon monoxide is also present in volcanoes.
Preparation: Carbon monoxide is produced from the incomplete combustion of carbon, the decomposition of organic compounds or though the reduction of carbon dioxide. Through these processes is produced all the carbon monoxide found in atmosphere. Carbon monoxide can also be produced by the oxidation of hydrocarbon gases that are present in the natural gas.
Physical properties: Carbon monoxide is a colourless, odourless gas. The density of this gas is 0.79 g/mL (liquid) and 1.145 g/mL (gas). Its melting point is -205 °C and the boiling point is -191.5 °C. It is largely soluble in water and other solvents as chloroform, acetic acid, ethanol, methanol, toluene and ammonium hydroxide.
Chemical properties: Carbon monoxide is a stable molecule due to the strength exhibits by the triple bond. The strong dipole moment due to the difference of electronegativity between the oxygen and carbon create a partial charge of -1 over the oxygen and +1 over the carbon atom. This fact along with the similar size between both atoms also contributes with the stability and low reactivity of the molecule.
Uses: Carbon monoxide can be used in chemical industry as a reducing agent. It is also a reagent in some organic synthesis reactions as the Frischer-Tropsh process. Other uses include the formation of chemical intermediaries through reactions that use carbon monoxide or end products as methanol, ethylene and acrylates. Carbon monoxide is also present in some mixtures of fuel.
Health effects / safety hazards: Carbon monoxide is is fatal by inhalation. This gas is extremely toxic and causes dozens of fatal air poisoning. It replaces the position of the oxygen in the haemoglobin, producing carboxyhemoglobin causing asphyxia. Carbon monoxide also ignites easily, causing flame. It is flammable.
|
Related Links: |
