Calcium carbonate Formula
Calcium carbonate is chemical compound largely used as additive in agriculture and it is also sold as a calcium supplement.
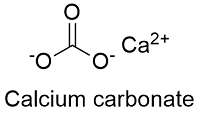
Formula and structure: The calcium carbonate chemical formula is CaCO3 and its molar mass is 100.0869 g mol-1. The molecule is formed by the calcium cation Ca+2 and the carbonate anion CO3-2. The structure of the calcium carbonate lattice depends on the mineral from it is extracted: the calcite contains a hexagonal calcium carbonate structure while the aragonite contains orthorhombic lattice. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Calcium carbonate is frequently found in nature as a component of rocks, corals, pearls, marine organism shells, eggshell and snailshell. It is also found in geological formations in the mineral calcite, dolomite, varite and aragonite or lime. It is also found dissolved in hard water.
Preparation: Most of the calcium carbonate is extracted from the minerals calcite and aragonite. Although it can also be produced by the reaction between the calcium hydroxide and carbon dioxide, using as starting material calcium oxide, which is transformed into calcium hydroxide by reaction with water.
CaO + H2O → Ca(OH)2 + H2O → CaCO3
Physical properties: Calcium carbonate is an odorless, tasteless, white and fine powder. Its density is 2.711 g mL. Calcium carbonate melting is 1339 ºC and above this temperature it decomposes. It is poorly soluble in water but it is soluble in dilute acids.
Chemical properties: Calcium carbonate causes temporary hardness in water together the magnesium carbonate. Similar to calcium sulfate, calcium carbonate is poorly soluble in water due the calcium cation (from the second group of periodic table) is voluminous and forms a molecule that is inefficiently solvate by water molecules. When it is in water, is formed an equilibrium between the carbonated dissolved and the solid (reaction I). If the water is satured with carbon dioxide, occurs a reaction yielding to calcium bicarbonate (reaction II):
CaCO3 ⇌ Ca2+ + CO32−
Calcium carbonate suffer analogous reaction to carbonate when it react with acids, releasing carbon dioxide:
CaCO3(s) + 2H+(aq) → Ca2+(aq) + CO2(g) + H2O (l)
CaCO3 + CO2 + H2O → Ca(HCO3)2
Uses: Calcium carbonate has many applications in industry and directly as drug. Similar to other calcium compounds and carbonates of group two metals, it is mainly used to obtain building materials as stucco and cement. Calcium carbonate is also used in the production of sugar from beet and in the chalk used in blackboard. It is also an additive in diapers, coats and paints and in some polymers, ceramics and adhesives, where it is added to increase the rigid. Additionally, calcium carbonate is largely used in agriculture and medicine as a calcium supplement and in dialysis for renal diseases. It is also used as excipient in the pharmaceutical industry.
Health effects / safety hazards: Calcium carbonate cause skin irritation and when it is in large quantities can cause serious eye damage. It is not flammable.
|
Related Links: |
