Calcium Bromide Formula
Calcium bromide, also knowns as calcium dibromide, is a chemical compound known for being used in some drilling fluids and food preservatives.
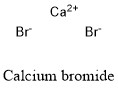
Formula and structure: Calcium bromide chemical formula is CaBr2 and its molar mass is 199.89 g mol-1. The compound is commonly found as a hydrated salt with 2 molecules of water and a molar mass of 235.98 g mol-1. It can also be found as a hexahydrate salt. The structure of the anhydrous salt is formed by one cation Ca2+ and two anions Br-. The structure is an octahedron with Ca centers bound to a total of six anion Br- through ionic bonds. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Calcium bromide is not found in nature.
Preparation: Calcium bromide is prepared from the reaction of calcium oxide with hydrobromic acid. Alternatively, it can be prepared with the reaction of metallic calcium and elemental bromine:
CaO + 2 HBr → CaBr2 + H2O
Ca + Br2 → CaBr2
Physical properties: Calcium bromide is a white hygroscopic powder in its anhydrous form. Its density is 3.35 g mL-1. The melting point of the anhydrous salt is 730 °C, while and the boiling point is 1935 °C. Calcium bromide is soluble in water, ethanol, methanol and acetone. It is insoluble in organic solvents.
Chemical properties: Calcium bromide can suffer different reactions. The most known and useful for the chemical industry is the formation of calcium oxide from calcium bromide and oxygen gas:
2 CaBr2 + O2 → 2 CaO + 2 Br2
This reaction is performed at high temperatures and bromine gas is formed as a by-side product.
Uses: Calcium bromide can be used by the food industries as a preservative and as a component of freezing mixtures. It is used as a fire retardant and as a wood preservative. Calcium bromide can also be used as a dehydrating agent.
Health effects / safety hazards: Calcium bromide can irritate eyes, skin and mucous. It is toxic by ingestion. Calcium bromide is not combustible or flammable.
|
Related Links: |
