Benzoic Acid Formula
Benzoic acid, also known as arboxybenzene or dracyclic acid, is an organic acid largely used in the food preservation and as intermediate in industrial synthesis.
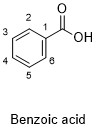
Formula and structure: The benzoic acid chemical formula is C7H6O2. The molar mass is 122.12 g/mol. The extended formula is C 6H5COOH and it is formed by an aromatic ring, in which is bond a carboxyl group COOH. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Benzoic acid can occur in plants, where is a intermediate to form many secondary metabolites.
Preparation: Benzoic acid can be prepared using different methods and strategies:
a) An oxidation of the solvent toluene with oxygen (O2). This reaction is a liquid phase reaction and takes place at 165 °C. In this process is also used cobalt as catalyst.
b) Decarboxylation of the phthalic anhydride and using zinc oxide as catalysts.
c) Hydrolysis of benzotrichloride to benzoic acid.
Physical properties: Benzoic acid is a white crystalline solid with a faint odor. The density of this solid is 1.266 g/mL. Its melting point is 122 °C and its boiling point is 250 °C. Benzoic acid solubility in water increases with the increment of temperature. It is soluble in many solvents such as methanol, ethanol, ethyl acetate, benzene, acetone, carbon tetrachloride and chloroform.
Chemical properties: Benzoic acid molecule has two functional groups that can suffer chemical reactions. These groups are the aromatic ring (-C6H5) and the carbonyl group (-COOH). The aromatic ring can suffer a substitution in its position meta. This substitution is known as electrophilic aromatic substitution and it is lead by the presence of the carbonyl group, which is EWG (electro-withdrawing group) that makes the meta position deficient in electron and thus, it can attack an electrophile. The carbonyl group is very versatile and it can react to the formation of esters, amides, halogenations, etc.
Uses: Benzoic acid has a large amount of applications in several industries. It is used as a raw material in the production of chemical compounds as phenol, benzoates, caprolactam and esters. It is used in the preservation of food such as fruits juices and fats. Benzoic acid is also used in the curing of tobacco and in the preparation of flavors and perfumes. In chemical synthesis, benzoic acid is used as a precursor in the synthesis of butyl benzoate and in the production of benzoyl chloride. In medicine, it Is used as a fungicide in the treatment of tinea and ringworm.
Health effects / safety hazards: Benzoic acid can cause skin irritation and serious eyes damage. It can be dangerous for the organs after a long-term exposition.
|
Related Links: |
