Barium Acetate Formula
Barium acetate, also known as barium salt of acetic acid or octan barnaty, is a salt used as a catalyst and also as a component of some pigments and paints.
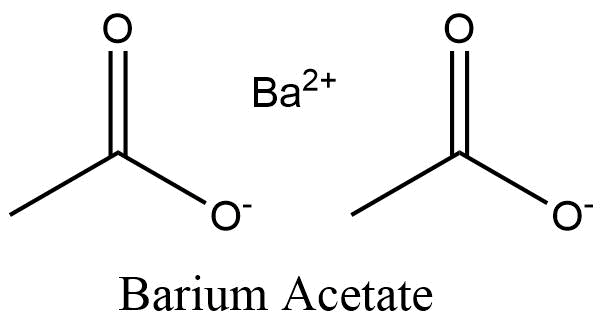
Formula and structure: The barium acetate chemical formula is C4H6BaO4. The molar mass is 255.42 g/mol. The condensed formula is Ba(CH3COO-)2 and is formed by one barium cation joined to two acetate anions CH3COO-. The structure of the acetate ion is planar due to the pi bond between C=O. The geometry of the molecule is planar. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Barium acetate is not found in nature. It is prepared from one of the methods described below.
Preparation: Barium acetate is prepared from two reactions. The first of these reactions os the reaction of barium carbonate with acetic acid, a very easy and cheap method to prepare the salt:
BaCO3 + 2CH3COOH → (CH3COO)2Ba + CO2 + H2O
The second method is through the reaction of the barium sulfide and acetic acid:
BaS + 2CH3COOH → (CH3COO)2Ba + CO2 + H2S
Physical properties: Barium acetate is a white, odorless solid. The density of this salt is 2.46 g/mL. Its melting point is 450 °C and above this temperature, it decomposes. It is very soluble in water. Barium acetate is slightly soluble in ethanol and it is insoluble in non-polar solvents.
Chemical properties: Barium acetate can decompose when heating to form barium carbonate. It can also be used to form different salts when used in reaction with acids:
(CH3COO)2Ba → H2O + BaCO3
(CH3COO)2Ba + H2SO4 → BaSO4 + CH3COOH
Uses: Barium acetate can be used to produce other barium components. It is also used in the preparation of other acetates. A second range of uses includes its use in the formulation of pigments, paints, dyes, coats. It can also be used as a catalyst in organic synthesis.
Health effects / safety hazards: Barium acetate is very toxic and can cause severe damage if swallowed or inhaled. It is not flammable.
|
Related Links: |
