Ammonium bicarbonate Formula - Ammonium bicarbonate Uses, Properties, Structure and Formula
Ammonium bicarbonate (commonly known as ammonium hydrogen carbonate) is a mildly basic inorganic compound.
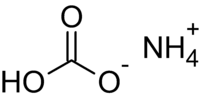
Formula and structure: The chemical formula of ammonium bicarbonate is NH4HCO3. Its molecular formula is CH5NO3 and its molar mass is 79.056 g/mol. Its chemical structure consists of the ammonium cation (NH4+) and the bicarbonate anion (HCO3-).
Occurrence: Ammonium bicarbonate is present in small quantities in nitrogenous organic matter, along with other ammonium salts.
Preparation: Ammonium bicarbonate is produced by passing carbon dioxide gas into aqueous ammonia until the ammonium bicarbonate crystals are formed, which are then separated from the solution by filtration or centrifugation. To prevent decomposition of the product, the reaction is performed at cold temperatures.
CO2 + NH3 + H2O → NH4HCO3
Physical properties: Ammonium bicarbonate exists as a white crystalline solid with a density of 1.59 g/mL and melting point of 41.9 °C. It has a strong odor of ammonia, and is highly water soluble.
Chemical properties: It dissolves in water to give a mildly alkaline solution. It is insoluble in most organic solvents. While it is stable at room temperature (25 °C), it decomposes at temperatures above 36 °C to form ammonia, carbon dioxide, and water in an endothermic reaction (absorbs energy for the reaction from the surroundings).
NH4HCO3 → NH3 + CO2 + H2O
It reacts with acids to produce carbon dioxide, and reacts with bases to produce ammonia.
Uses: Ammonium bicarbonate is used as a baking powder, in some food processing applications, in cough syrups and as antacid. It also has uses as a fertilizer, pH buffer, and reagent in chemical laboratories. In the industry, it is used in the manufacture of dyes, pharmaceuticals, catalysts, ceramics, fire-retardants, plastics and other products.
Health effects/safety hazards: In low concentrations, it is not considered hazardous. Its main health hazard is its decomposition reaction giving pungent ammonia gas, which is a serious irritant. Inhalation of ammonium bicarbonate can irritate the eyes, skin, nose and entire respiratory system, and cause severe coughing and difficulty in breathing.
|
Related Links: |
