Ammonium acetate Formula
Ammonium acetate is salt that has interesting chemical properties and for that reason, it is very used in pharmaceutical industry as intermediary and raw material in diverse processes.
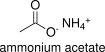
Formula and structure: Ammonium acetate chemical formula is NH4CH3CO2 or CH3COONH4. Its molecular formula is C2H7NO2 and its molar mass is 77.08 g mol-1. Ammonium acetate is the salt of acetate ion COO-1 (from acetic acid dissociation in water) and ammonium ion NH4+ (from ammonia dissociation in water). Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Ammonium acetate is not present as free-compound in nature. However, ammonium and acetate ions are in many biochemical processes.
Preparation: Ammonium acetate can be synthesized in a similar way to other acetates through neutralization of acetic acid. The synthesis uses acetic acid, which is neutralized adding ammonium carbonate. In chemical industries, the method uses glacial acetic acid that is saturated with ammonia:
2CH3COOH + (NH4)2CO3 → 2CH3COONH4 + H2CO3
H2CO3 → CO2 + H2O
CH3COOH + NH3 → CH3COONH4
Physical properties: Ammonium acetate is a hygroscopic white solid with a slightly acetid odor. Its melting point is 113 ºC. It is highly soluble in water and its density in this liquid is 1.17 g mL-1.
Chemical properties: Ammonium acetate is a salt of a weak acid (acetic acid) and a weak base (ammonia). This salt is used with acetic acid to prepare buffer solution to regulate pH. However, its use as buffering agent is not very extended due ammonium acetate can be volatile in low pressures.
Uses: Ammonium acetate is used as a raw material in synthesis of pesticides, herbicides and non-steroidal anti-inflammatory drugs. Additionally, it is the precursor in the acetamide synthesis; a chemical compound used to produce plasticizers:
CH3COONH4 → CH3C(O)NH2 + H2O
Ammonium acetate is used to acidify textiles and hair dyes and in some countries, it is used as food acidity regulator. It is a buffering agent with acetic acid. Moreover, ammonium acetate has found application as catalyst in organic chemistry, in reactions such as Knoevenagel condensations. It can be used as fertilizer and in synthesis of explosives.
Health effects/safety hazards: Ammonium acetate is an irritant of eyes, mouth, nose and skin. It is highly dangerous by ingestion and can cause tissue necrosis. It can saponify the skin, destroying the cell membranes and penetrating into organism. Ammonium acetate produces toxic fumes when heated. It causes the decomposition of sodium hypochlorite in a few seconds.
|
Related Links: |
Related Topics
Ionic and Net Ionic Equations
