Aluminium hydroxide Formula
Aluminium hydroxide is a inorganic basic compound used as intermediary in organic synthesis and as additive in pharmaceutical and fine chemical industries.
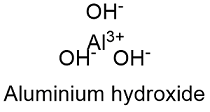
Formula and structure: The aluminium hydroxide chemical formula is Al(OH)3 and its molar mass is 78.00 g mol-1. The molecule is formed by the aluminium cation Al+3 and three hydroxyl anions CO3-2. The structure of the aluminium hydroxide lattice depends on the mineral from it is extracted because the ions show different arrangements. Most of the lattice are hexagonal or orthorhombic. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Aluminium hydroxide, similar to other metals carbonates, hydroxides and sulfates, is found in mineral ores of gibbsites, bayertute, doyleite and strandite.
Preparation: Although aluminium hydroxide is largely found in many geological systems in nature, it is mostly produced by the Bayer and sintering processes to obtain alumina from the mineral bauxite. Thus, 97% of the world aluminium hydroxide is obtaining through the treatment of bauxite with caustic soda yielding sodium aluminate, which is decomposes by stirring to obtain an aluminium hydroxide precipitate:
NaAl(OH)4 → Al(OH)3 + NaOH
Other processes to obtain aluminium hydroxide are the hydrothermal technique, the micro-emulsion or the Sol-gel. These methods have the advantage of producing an compound with a higher level of purity.
Physical properties: Aluminium hydroxide is an odorless, white amorphouse solid. Its density is 2.42 g mL-1. Aluminium hydroxide melting point is 300 ºC. It is insoluble in water and ethanol, but soluble in acids and alkalis solutions.
Chemical properties: Aluminium hydroxide is an amphoteric compound, which means that the substance presents basic or acid characteristics. Consequently, the aluminium hydroxide is soluble in both: acids (reaction I) or alkalis (reaction II) solutions:
Al(OH)3 + 3 H+ → Al+3 + H2O (I)
Al(OH)3 + OH- → AlO2- + H2O (II)
Uses: Aluminium hydroxide has a great variety of application in chemical industry, some of these uses are as plastic, rubber, polymer and epoxy resin filler, flame retardant, additive for glass and paper. In pharmacy, it is used as an antacid for the gastritis and ulcer treatment and it is also an additive in some vaccines and works as excipient in the production of some drugs. However, aluminium hydroxide is mostly used as raw material in the production of alumina (aluminium oxide) to produce aluminium metal.
Health effects / safety hazards: The aluminium hydroxide in high concentration causes serious damage to the health. It may cause damage in lungs. It is not flammable.
|
Related Links: |
