Aluminium dioxide (alumina) Formula
Aluminium dioxide, also known as aluminium (III) oxide, is an oxide found in minerals as bauxite and corundum. Alumina is commonly used as catalyst and adsorbent.
Formula and structure: The aluminium oxide chemical formula is Al2O3. Its molecular weight is 101.96 g mol-1. This oxide is formed by the cation aluminum Al+3 and the anion oxygen O-2. Due Al2O3 is extracted from different mineral in nature, there is not a unique structure for fitting the molecules in this compound, so it can have octahedral, hexagonal or cubic geometry. The most common geometry is octahedral structure. Its chemical structure can be written as below, in the common representations used for organic molecules.
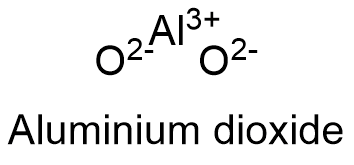
Occurrence: Aluminium oxide can easily be found in nature in ores of corundum and bauxite spread around the planet, mainly in Greece, Guinea and Australia.
Preparation: Aluminium oxide is extracted from mineral through addition of sodium hydroxide to the minerals in a process called The Bayer Process. The first reaction between the mineral and sodium hydroxide produces sodium aluminate solution, which is cooled to precipitate aluminum hydroxide. This compound is calcined over 1100 ºC to obtain aluminium oxide:
Al2O3 + H2O + NaOH → NaAl(OH)4
Al(OH)3 + NaOH → NaAl(OH)4
NaAl(OH)4 → NaOH + Al(OH)3 (s)
2 Al(OH)3 → Al2O3 + 3 H2O
Physical properties: Aluminium oxide is a white, odorless, crystalline solid. Its boiling and melting points are very high: 3000 ºC and 2054 ºC, respectively. Aluminium oxide density is 3.97 g mL-1. It is not soluble in water.
Chemical properties: Aluminium oxide is an amphoteric oxide, so that it can react with acids or bases. In both cases, the reaction leads to a salt of aluminum:
Al2O3 + 6 HCl → 2 AlCl3 + 3 H2O
Al2O3 + 2 NaOH + 3 H2O → 2 NaAl(OH)4
Uses: Aluminium oxide is extensively used as intermediate to produce: building and construction materials, electronic and electric devices, metals, glass paints (cosmetic and automotive industries) and coatings. It is very used in the oil industry and also to dried compound for removing water in industrial facilities. In chemical industry, aluminium oxide is used as catalyst to promote reaction such as dehydration of alcohol or hydrodesulfurization.
Health effects/safety hazards: Aluminium oxide may cause damages to eyes. It can also be an irritating to respiratory system. The long term exposure to aluminium oxide may affect the central nervous system. It is not flammable, however may form explosive mixture in contact with air.
|
Related Links: |
