Activation energy Formula
Definition: Activation energy is the minimum energy that a system must have for allowing the molecules' reactions to the expected products. The concept was proposed by the Swedish chemist Svante Arrhenius in 1888 and it is measured in the International system units used for energy Joule (J) or kilojoules per mole (kJ mol-1). Additionally, it can be measured in kilocalories per mole (kcal.mol-1).
According to the concept of activation energy, every reaction has a potential barrier or minimal energy that should have the molecules in order to stretch, bend or break bonds for forming the products of the reaction. This energy comes from the kinetic energy produces by the molecules that are colliding all the time. If the temperature of the system is high, more collision will take place and more kinetic energy will be available for reaching the activation energy.
Formula: The activation energy formula is derived from the Arrhenius equation:
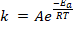 or
or 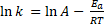
Where A is the pre-exponential factor for the reaction (that is nearly a constant that depends on the temperature), Ea is the activation energy, R is the gas constant, T is the temperature and k is the reaction rate constante. In this equation Arrhenius incorporated three factors: a) the molecules that have the potential energy required for reacting, 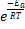 , b) the collisions between molecules' factor and c) the number of collisions that have the appropriate orientation factor, both expressed by the pre-exponential factor A.
, b) the collisions between molecules' factor and c) the number of collisions that have the appropriate orientation factor, both expressed by the pre-exponential factor A.
Use: The activation energy factor is fundamental for theoretical calculates related to the use of catalysts or for modeling some biological systems as the enzyme-substrate systems. A catalyst has the functions of lowing the activation energy for allowing that more molecules reach it and also increase the rate constant of the reaction. These systems can be modeled using activation energy equation.
Example: Determine the activation energy given of the next reaction, considering as T 400 K and k = 2.75 x 10-2 L mol-1 s-1 and pre-exponential factor is 20 M-1s-1.
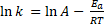 , (ln A - ln k) RT= Ea
, (ln A - ln k) RT= Ea
R is gas constant (8.314 J/ molK), then
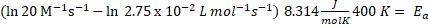
Ea = 21.91 kJ/mol
Considerations : In the Arrhenius equation, we can see if the magnitude of Ea increases, the rate constant of the reaction k decreases because fewer molecules will have the enough energy to reach the potential energy and react.
|
Related Links: |
