Oxidation Reactions
Oxidation, oxidation-reduction, or redox reactions are the terms used to describe a chemical reaction where one atom increases its oxidation state, while another atom has a reduction in oxidation state. The change in oxidation state mainly occurs due to a transfer of electrons during the chemical reaction. The element which loses electrons has a corresponding increase in oxidation, while the element which gains electrons has a reduction.
Organic oxidation and reduction reactions focus on the organic molecule in the reaction, and more specifically the carbon that they contain. The term oxidation and reduction are used as the oxidation state of the carbon atom changes in the reaction. Organic redox is not always accompanied by electron transfer, which is different from most redox reactions.
1. Reduction of an alkene to an alkane:
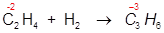
In this example the oxidation state of the carbon atom is reduced from -2 to -3 during the course of this addition reaction.
2. Combustion of a hydrocarbon:
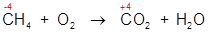
In this example the oxidation state of the carbon atom is increased from -4 to +4.
Organic oxidation and reduction reactions focus on the organic molecule in the reaction, and more specifically the carbon that they contain. The term oxidation and reduction are used as the oxidation state of the carbon atom changes in the reaction. Organic redox is not always accompanied by electron transfer, which is different from most redox reactions.
1. Reduction of an alkene to an alkane:
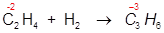
In this example the oxidation state of the carbon atom is reduced from -2 to -3 during the course of this addition reaction.
2. Combustion of a hydrocarbon:
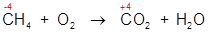
In this example the oxidation state of the carbon atom is increased from -4 to +4.
|
Related Links: Chemistry Organic Chemistry Stereoisomers Addition Reactions |
To link to this Oxidation Reactions page, copy the following code to your site:
