Hydrogen Bonding
Forces of attraction that draw molecules together are called intermolecular forces. The three main intermolecular forces are London dispersion forces, dipole-dipole interactions and hydrogen bonding. Hydrogen bonds are basically very strong dipole-dipole interactions in which two polar molecules attract to each other.
In a hydrogen bond, the negative end of one molecule will attract to the positive end of a nearby molecule. The reason that hydrogen bonds are more effective than a dipole-dipole interaction is due to the hydrogen atom being attached to a very electronegative (N, O, F) atom in one of the molecules. Because hydrogen is so small the polar molecules can get closer together where the attraction is greater.
Examples:
1. The hydrogen bonding in HF.
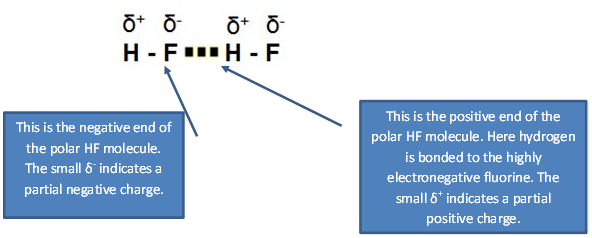
2. The hydrogen bonding in H2O.
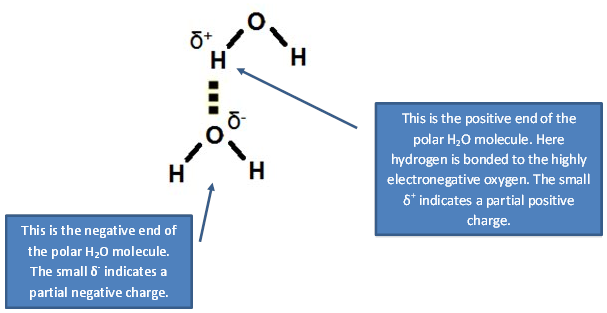
In a hydrogen bond, the negative end of one molecule will attract to the positive end of a nearby molecule. The reason that hydrogen bonds are more effective than a dipole-dipole interaction is due to the hydrogen atom being attached to a very electronegative (N, O, F) atom in one of the molecules. Because hydrogen is so small the polar molecules can get closer together where the attraction is greater.
Examples:
1. The hydrogen bonding in HF.

2. The hydrogen bonding in H2O.

|
Related Links: Chemistry Organic Chemistry Intermolecular Forces Mass Spectroscopy |
To link to this Hydrogen Bonding page, copy the following code to your site:
