Alkanes
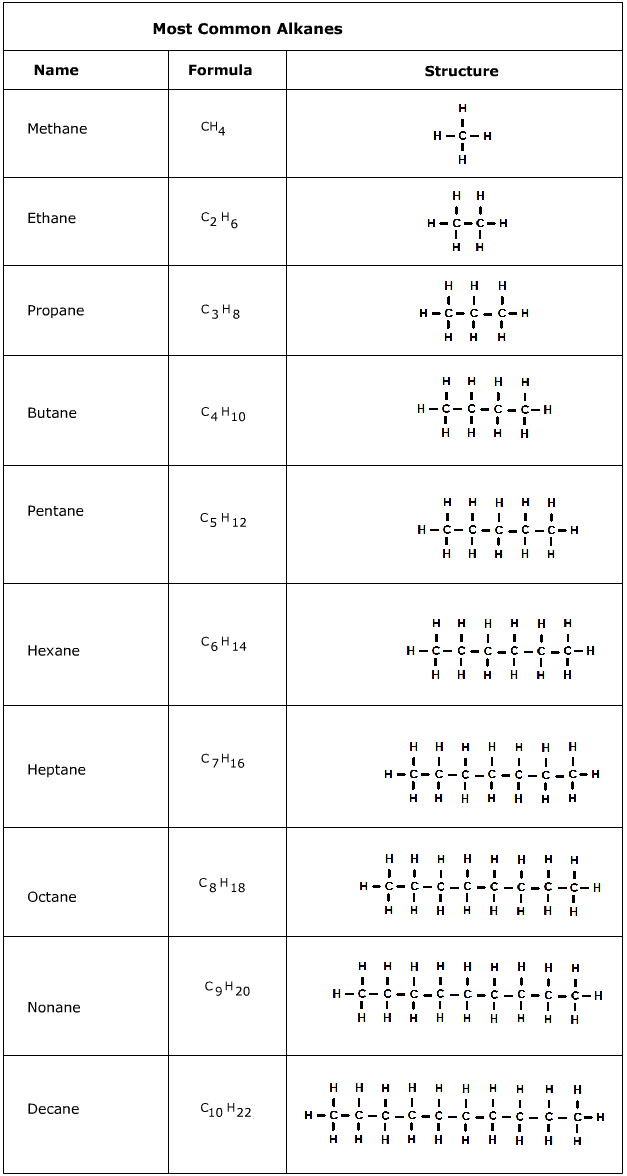
Alkanes are one of many groups of organic molecules. They are hydrocarbons, which mean they consist of only the elements carbon and hydrogen. The distinct feature of alkanes is that they are saturated hydrocarbons, referring to the fact that each carbon contains the maximum number of hydrogens. A simpler way to describe this is that alkanes only contain single bonds. This leads to the general formula for alkanes, CnH2n+2, where n is any integer. For example an alkane with n = 2 carbon atoms, will have a formula of C2H6.
Alkanes can come in many shapes and sizes. The most common type of alkane is a straight chained alkane where the carbon atoms connect in a row. Branched chain alkanes have carbon atoms that branch off of the longer row of carbon atoms. Cycloalkanes form rings of carbon atoms with a general formula of CnH2n.
Alkanes are used in a variety of materials, but their main use is for energy. Natural gas, propane (gas grills), butane (cigarette lighters), and octane are all alkanes that have use as a fuel. Longer chain alkanes can be used in candles. The stored energy in the carbon to hydrogen bonds is where the potential is for hydrocarbon use as a fuel source.
Alkanes can come in many shapes and sizes. The most common type of alkane is a straight chained alkane where the carbon atoms connect in a row. Branched chain alkanes have carbon atoms that branch off of the longer row of carbon atoms. Cycloalkanes form rings of carbon atoms with a general formula of CnH2n.
Alkanes are used in a variety of materials, but their main use is for energy. Natural gas, propane (gas grills), butane (cigarette lighters), and octane are all alkanes that have use as a fuel. Longer chain alkanes can be used in candles. The stored energy in the carbon to hydrogen bonds is where the potential is for hydrocarbon use as a fuel source.

|
Related Links: Chemistry Organic Chemistry Alkenes |
To link to this Alkanes page, copy the following code to your site:
