Alpha Decay
Radioactive decay involves the spontaneous splitting of heavy unstable isotopes. The isotope splits to create two or more stable particles. One of the three main types of radioactive decay is known as alpha decay (α-decay).
An alpha particle is a name given to a particle that contains two protons and two neutrons. It is identical to a helium nucleus (no electrons). In alpha decay, the unstable isotope will emit an alpha particle, along with a more stable isotope (or isotopes). The masses of the elements are conserved during alpha decay.
Examples:
1. The alpha decay of platinum-175
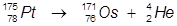
In this reaction, platinum-175 undergoes α-decay to produce osmium-171.
2. The alpha decay of uranium-238
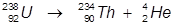
In this reaction, uranium-238 undergoes α-decay to produce thorium-234.
An alpha particle is a name given to a particle that contains two protons and two neutrons. It is identical to a helium nucleus (no electrons). In alpha decay, the unstable isotope will emit an alpha particle, along with a more stable isotope (or isotopes). The masses of the elements are conserved during alpha decay.
Examples:
1. The alpha decay of platinum-175
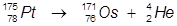
In this reaction, platinum-175 undergoes α-decay to produce osmium-171.
2. The alpha decay of uranium-238
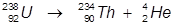
In this reaction, uranium-238 undergoes α-decay to produce thorium-234.
|
Related Links: Chemistry Nuclear Chemistry Beta Decay Gamma Decay Nuclear Fusion Nuclear Reactions |
