Enduring Understanding 4.D: Catalysis
- Catalysts are substances that increase the rate of a chemical reaction.
- Examples of catalysts are enzymes in biological systems, and catalytic converters in exhaust systems.
- Catalysts work by lowering the activation energy of the rate-determining step of a reaction - either by stabilizing the transition state of an existing elementary step in the reaction pathway, or by providing a different, lower energy pathway for the reaction to occur.
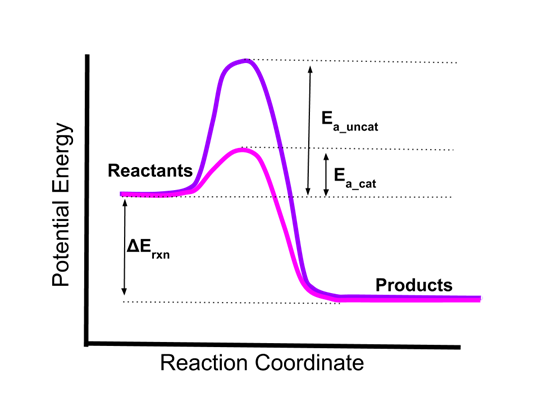
- Here is an energy diagram of a single step reaction in the absence and presence of a catalyst.
- The uncatalyzed reaction (in purple) has a high activation energy, Ea_uncat.
- The catalyzed reaction (in pink) has a greatly lowered activation energy, Ea_cat.
- Because of this lowering of the activation energy, the reaction will proceed much more quickly in the presence of the catalyst.
- Note that the thermodynamic energy change of the reaction, Erxn, does not change. Catalysis does not change the overall energetics of the reaction.
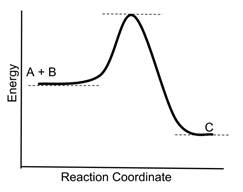
- Sample Question: Consider the following reaction coordinate diagram for the reaction A + B → C
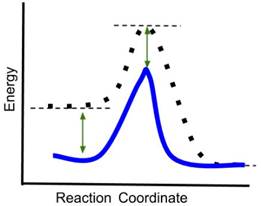
- If a catalyst is added to the reaction, which new reaction coordinate diagram below will best represent the reaction? The new energy curves are colored; the original curve is dotted. Green arrows indicate changes in energy.
- Answer: (d) A catalyst decreases only the energy of the transition state of a reaction, thereby reducing the activation energy and increasing the rate of the reaction. It does not change the energy of the reactants or products. In the above graphics, (a) reduces the energy of the reactants and transition state, (b) reduces the energy of the transition state and the product, (c) reduces the energy of the reactants, transition state and product. Only (d) reduces only the energy of the transition state.
a)
 b)
b)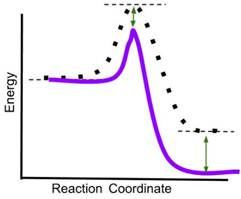
c)
 d)
d)
To link to this Catalysis page, copy the following code to your site:
