Zinc Sulfide Formula
Zinc sulfide is an inorganic compound which has useful optical properties.
Formula and structure: The chemical formula of zinc sulfide is ZnS, and its molar mass is 97.47 g/mol. The chemical structure of ZnS is simple and consists of the zinc metal attached to a sulfur atom through a polar covalent bond.
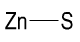
Solid zinc sulfide is found in two different crystal forms, alpha (wurtzite) and beta (sphalerite), which have hexagonal and cubic structures, respectively. The beta crystalline (sphalerite) is the more stable form of ZnS.
Occurrence: Zinc sulfide occurs naturally as the mineral zinc blende, also called sphalerite, which is a mixture of iron and zinc sulfides.
Preparation: Zinc sulfide is commonly prepared through several simple reactions, such as the combustion of a mixture of zinc and sulfur, reacting zinc sulfate (ZnSO 4) with sodium sulfide (Na2S), or by passing hydrogen sulfide (H2S) gas into aqueous solution of any Zn2+ salt to precipitate the insoluble ZnS. It is also prepared by reacting zinc oxide with hydrogen sulfide:
ZnO + H2S → ZnS + H2O
Physical properties: Zinc sulfide is found in two different crystal forms, as mentioned above. The wurtzite form exists as white or yellowish-white crystals, while the sphalerite form exists as greyish white crystals. It has a density of 4.09 g/mL, and melting point of 1,185 °C.
Chemical properties: Zinc sulfide is completely insoluble in water. It decomposes in the presence of acids and strong oxidizing agents. At temperatures higher than 900 °C, it decomposes with the release of zinc and sulfur fumes, and it also reacts with strong acids to release hydrogen sulfide gas. When heated to a temperature of 102 °C, the stable beta crystalline form of ZnS (sphalerite) changes into the alpha form (wurtzite). ZnS is a luminescent material, and exhibits phosphorescence when illuminated with UV light.
Uses: Zinc sulfide has several applications due to its luminescent property. It is doped with different activators and used for making phosphorescent and electroluminescent materials. Zinc sulfide is used as a pigment, infrared optical material, and for making optical windows and lenses. It is also a wide-bandgap semiconductor and an efficient photocatalyst.
Health effects/safety hazards: Zinc sulfide is not very hazardous for humans and mainly causes irritation of skin, eyes and respiratory tract upon exposure. However, it is considered a serious environmental hazard as it is highly toxic to aquatic organisms.
|
Related Links: |
Related Topics
Chemistry Formulas
Formulas: Physics Formulas and Math Formulas
General Chemistry topics
Combustion Reaction Examples
Ionic and Net Ionic Equations
