Thiamine Formula
Thiamine or vitamin B1, is an macromolecule and chemical compound that is part of the World Health Organization model list of most essential medicines. It is essential to the conversion of carbohydrates and fat into energy.
Formula and structure: The thiamine chemical formula is C12H17N4OS+Cl- and its molar mass is 265.35 g mol-1.The molecule includes several functional groups: an aminopyridine (red colored) and thiazole (blue colored) ring, the molecule also has methyl and hydroxyethyl side chains. Its chemical structure can be written as below, in the common representations used for organic molecules.
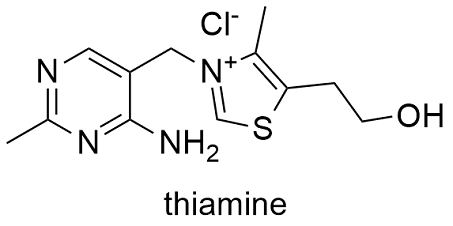
Occurrence: Thiamine is found in nature in many organisms, since bacteria to plants and fungi. It is biosynthesized by these micro-organisms and organisms through various enzymes.
Preparation: Even when thiamine is produced by many organisms, it is not found free in nature and it cannot be isolated easily from the producers. Thus, the organic synthesis has developed some methods to obtain thiamine. The main method is the built of the thiazole ring and then, it can react with the 4-amino-5-aminomethyl-2-methylpyrimidine ring to form the whole structure of thiamine. A second method, also use the thiazole ring but later, it reacts with 4-amino-5-bromomethyl-2-methylpyrimidine.
Physical properties: Thiamine is a colorless to white solid. Its density is 1.029 g mL-1 and its melting point is 250 ºC and a high temperatures, it decomposes. Thiamine is insoluble in ethanol. It is soluble in water, methanol and glycerol.
Chemical properties: Thiamine in nature is biosynthesized as a thiazol moiety and a pyrimidine moiety and then these are combined by enzymatic action to obtain the thiamine, an essential vitamin in many organisms and that acts as antioxidant and in many detoxification mechanisms. As an antioxidant, it has the role of control the quantity of free radicals, which affect the brain activity.
Uses: Thiamine is used in the pharmaceutical industry to produce thiamine supplements to treat its deficiency syndromes such as encephalopathy syndrome or delirium. It can also be used in the treatment of appetite and chronic diarrhea.
Health effects / safety hazards: Thiamine can cause skin irritation and severe damage to eyes. It may also cause respiratory tract irritation. It is not flammable and it does not react with other chemical compounds.
|
Related Links: |
