Tetramethylammonium hydroxide Formula
Tetramethylammonium hydroxide, also known by the name tetramethylazanium hydroxide or TMAH, is a quaternary ammonium salts largely used in chemical industry as raw material of industrial reactions.
Formula and structure: Tetramethylammonium hydroxide chemical formula is C4H3NO and its molecular mass is 91.15 g mol-1. The molecule is a salt, formed by the tetramethylammonium cation N(CH3)4+ and the hydroxyl anion OH-. The cation is derived from a quaternary amine, which has the minimal units of substitution (methyl groups), the geometry of this group is tetrahedral. Its chemical structure can be written as below, in the common representations used for organic molecules.
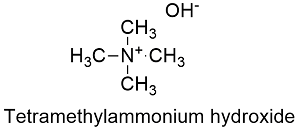
Occurrence: Tetramethylammonium hydroxide is not found in nature, it should be prepared through synthetic methods.
Preparation: Tetramethylammonium hydroxide is prepared followed a few methods, and the most extensively used is the reaction between tetramethylammonium chloride and potassium hydroxide in methanol:
NMe4+Cl- + KOH → NMe4+ OH- + KCl
And the tetramethylammonium chloride is synthesized by alkylation of ammonium chloride with dimethyl carbonate:
NH4+Cl- + 2 (CH3O)2CO → N(CH3)4+Cl- + 2 H2O + 2 CO2
Physical properties: Tetramethylammonium hydroxide is a solid when hydrated or a colorless to yellow liquid with a strong fishy smell. It is commonly found in solutions of water or methanol. The density is 1.015 g mL-1 and the melting point is 67 ºC and above this temperature, it decomposes.
Chemical properties: Tetramethylammonium hydroxide is a chemical compound very used in chemical industry due to the large quantityof reaction which can promote, for example:
Acid-Base reactions with acid or bases to form the tetramethylammonium salt corresponding (the processes is similar to the synthesis of TMAH from tetramethylammonium chloride):
NMe4+ OH- + HCl → NMe4+Cl- + H2O
NMe4+ OH- + NH4+SCN- → NMe4+SCN- + NH3 + H2O
Uses: Tetramethylammonium hydroxide is used as intermediate in many reactions to produce other tetramethylammonium salts. It is also used in chemical laboratories as reactant and precursor and in industries it is used as a solventto the acidic photoresist (during a process know as photolithography). Moreover, TAMH is catalyst of some reaction and is a reagent used in hermochemolysis.
Health effects / safety hazards: Tetramethylammonium hydroxide is extremely poisonous and it is fatal if swallowed or in contact with skin or mucous. It is also toxic to the aquatic life and it is corrosive to other compounds.
Not flammable or combustible.
|
Related Links: |
