Sulfurous acid Formula - Sulfurous Acid Uses, Properties, Structure and Formula
Sulfurous acid is a weak inorganic acid, which is considered an aqueous solution of sulfur dioxide in water.
Formula and structure: The chemical formula of sulfurous acid is H2SO3 and its molar mass is 82.07 g/mol. Its chemical structure is shown below. It consists of a sulfur atom having two single bonds with hydroxyl groups and one double bond with oxygen.
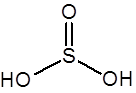
Occurrence: Sulfurous acid is found in nature as an intermediate in the formation of acid rain, by the reaction of sulfur dioxide with atmospheric moisture.
Preparation: Sulfurous acid is prepared by dissolving sulfur dioxide in water. However, the reaction is reversible and the acid readily decomposes back into the reactants.
SO2 + H2O → H2SO3
Thus, sulfurous acid is not usually available in its acid form, but more commonly prepared as its sodium or potassium salts. In the below reaction, sodium sulfate is added to a solution of sulfur dioxide in water, to give the stable sodium bisulfite salt as product.
Na2SO3 + H2O + SO2 → 2NaHSO3
Physical properties: Sulfurous acid is a colorless liquid with a strong pungent odor. It has a density of 1.03 g/mL, and a boiling point of -60 °C.
Chemical properties: Sulfurous acid is unstable, and has never been isolated in its pure state. It decomposes readily into water and sulfur dioxide:
H2SO3 (aq) → H2O + SO2
It also forms sulfuric acid when exposed to air:
2H2SO3 + O2 → 2H2SO4
Sulfurous acid is a weak and dibasic acid. It reacts with bases to form bisulfite and sulfite salts.
Uses: Sulfurous acid and its salts are used as powerful reducing agents and disinfecting agents. It is also used as a mild bleaching agent for applications having chlorine sensitive materials.
Health hazards/ health effects: Sulfurous acid itself is not commercially available as a free agent, and thus its health hazards are not severe on its own. However, it readily decomposes to release sulfur dioxide gas, which is toxic. Moreover, it forms sulfuric acid when exposed to air, which is a toxic, strong and corrosive acid. Therefore, inhalation, ingestion or skin contact with sulfurous acid may cause severe burns, eye injury, and respiratory problems.
|
Related Links: |
