Sodium hydride Formula - Sodium hydride Uses, Properties, Structure and Formula
Sodium hydride is a highly reactive inorganic hydride used as a strong base.
Formula and structure: The chemical formula of sodium hydride is NaH, and its molar mass is 24.0 g/mol. NaH is an ionic compound as shown below, and is made of sodium cations (Na+) and hydride anions (H-). It has the same octahedral crystal structure as NaCl, with each sodium ion surrounded by six hydride ions. The free hydride ions give this molecule its strong basic character.
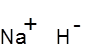
Preparation: Sodium hydride is prepared by the direct reaction between sodium metal and hydrogen gas.
2 Na + H2 → 2 NaH
Physical properties: Pure NaH is a colorless or white solid with a density of 1.4 g/mL, and a melting point of 800 °C. However, due to its reactivity, it is typically available as a grey solid dispersed in a mineral oil (60% weight by weight) for safe handling.
Chemical properties: Pure NaH can readily ignite in air. When it comes into contact with water present in the air, it releases highly flammable hydrogen gas. When open to air and moisture, NaH also gets easily hydrolyzed into the strong corrosive base, sodium hydroxide (NaOH). Thus, to prevent fires and explosions, sodium hydride is sold as a dispersion in mineral oil, allowing it to be safely handled in air.
NaH + H2O → NaOH + H2
Sodium hydride is a strong base commonly used in organic chemistry, where it is kept in an inert atmosphere (with 'dry' gases such as argon, nitrogen, etc.). It is insoluble in organic solvents.
Uses: Despite its dangerous reactivity, NaH is widely used as a powerful deprotonating and reducing agent for many organic reactions. It can also be used as a desiccant or drying agent for laboratory chemicals. Due to its ability to release hydrogen gas, it is being explored as a hydrogen storage agent in fuel cell vehicles.
Health effects/safety hazards: The main hazards from sodium hydride are its flammability and violent, explosive reaction with air and water. Moreover, it also forms the highly flammable hydrogen gas and strong base, NaOH, which can lead to further danger. Contact with sodium hydride by itself can burn eyes and skin. Exposure to sodium hydride dust can severely irritate the eyes, skin and respiratory system. It is extremely harmful if swallowed.
|
Related Links: |
