Sodium fluoride Formula - Sodium Fluoride Uses, Properties, Structure and Formula
Sodium fluoride is an inorganic salt, which is an important source of the fluoride ion for many applications.
Formula and structure: The chemical formula of sodium fluoride is NaF and its molar mass is 41.99 g/mol. It is a simple ionic compound, made of the sodium (Na+) cation and fluoride (F-) anion. The solid salt exists as cubic crystals similar to the crystal structure of sodium chloride (NaCl).
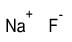
Occurrence: Sodium fluoride occurs in nature as the rare mineral villiaumite, in very small quantities.
Preparation: Industrial production is the major source of sodium fluoride. It is commonly prepared by neutralizing hydrofluoric acid with bases such as sodium carbonate (soda ash, Na2CO3), sodium hydroxide (caustic soda, NaOH) or sodium bicarbonate (NaHCO3).
HF + NaOH → NaF + H2O
Physical properties: Sodium fluoride is found as an odorless, crystalline solid that is white to greenish in color, depending on its purity. Its density is 2.56 g/mL, melting point is 993 °C, and boiling point is 1,704 °C. It is a hygroscopic solid (absorbs moisture from air).
Chemical properties: Sodium fluoride is highly soluble in water and readily dissociates into the sodium and fluoride ions in solution. It is noncombustible and corrosive to aluminum metal. It is stable under normal conditions, but when heated to high temperatures, it decomposes with the release of toxic and corrosive fumes of hydrogen fluoride (HF).
Uses: Sodium fluoride is widely used as a fluorinating agent in toothpastes and water supplies to maintain dental health. It is also used as a wood preservative, corrosion inhibitor, cleaning agent, insecticide, chemical reagent, and in metallurgy and glass industries. Another important application is in nuclear medical imaging, where radioactive sodium fluoride (NaF18) is used as a radiotracer for detecting various conditions.
Health effects/safety hazards: Sodium fluoride is toxic and corrosive. If swallowed, it can cause abdominal pain, vomiting, diarrhea, convulsions, collapse and even lead to death. Inhalation can cause severe irritation of the respiratory tract. Skin or eye contact with solid NaF may cause serious irritation or burns to skin and eyes, severe injury or even death.
|
Related Links: |
