Sodium cyanide Formula
Sodium cyanide is a chemical compound mostly known for being highly poisonous but very useful in organic synthesis and industrial processes.
Formula and structure: The sodium cyanide chemical formula is NaCN and its molar mass is 49.0072 g mol-1. The molecule is formed by the sodium cation Na+ and the cyanide anion CN- which is a linear ion formed by a carbon and a nitrogen atoms bound by a triple bond of 180º. The structure is highly similar to sodium chloride structure, with an anion CN- surrounded by 6 sodium cations. Its chemical structure can be written as below, in the common representations used for organic molecules.
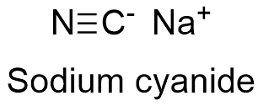
Occurrence: Sodium cyanide can be found in nature, mostly it is produced by some bacteria, fungi, algae and plants. Thus, this compound can be found in some foods such as almonds, beans, soy, spinach and cassava roots.
Preparation: Sodium cyanide is mostly obtained by the reaction between sodium amide with carbon at high temperature, which produces sodium cyanide and water:
NaNH2 + C → NaCN + H2
Sodium cyanide can also be prepared from melting sodium chloride and calcium cyanamide, or through the reaction between hydrogen cyanide with sodium hydroxide:
HCN + NaOH → NaCN + H2O
Physical properties: Sodium cyanide is a white crystalline solid with a faint almond-like odor. It is hygroscopic. Its density is 1.596 g mL-1. Sodium cyanide melting and boiling points are 563 ºC and 1496 ºC, respectively. It is soluble in water, ammonia, methanol and ethanol. It is isoluble in dimethylsulphoxide.
Chemical properties: Sodium cyanide is used in many organic and inorganic syntheses. It is frequently used to substitute some group by the -CN anion, which can be easily converted to other functional group more useful, such as aldehyde, ketone, etc. The main problem with sodium cyanide is the formation of a very poisonous HCN (hydrogen cyanide gas) when it reacts with acids (weak or strong acids).
NaCN + H2SO4 → HCN + NaHSO4
Uses: Sodium cyanide is used in chemical industry to promote some reaction, especially to manufacture plastic and other polymers but can also be used to manufacture cyanuric chloride or nitriles. It is also used in metallurgy and in a process known as cyanide mining to extract gold and silver in mining industry. The base of this process is the high affinity of metals for cyanide ion and producing the sodium metal cyanide:
4 Au + 8 NaCN + O2 + 2 H2O → 4 Na[Au(CN)2] + 4 NaOH
Health effects / safety hazards: Sodium cyanide is classified as a very dangerous substances because the reaction between this salt and weak or strong acids produce hydrogen cyanide gas, which is a deadly poison. It is introduced to the organism by ingestion or through the skin or inhalation. It is not flammable.
|
Related Links: |
