Silver Nitrate Formula
Silver nitrate is an inorganic silver compound which is used to prepare many types of silver derivatives.
Formula and structure: The chemical formula of silver nitrate is AgNO3, and its molar mass is 169.87 g/mol. It is a salt, and its chemical structure consists of the silver cation (Ag+) and the nitrate ion (NO3-), in which the central nitrogen atom is covalently bonded to three oxygen atoms with a net charge of -1.
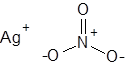
Preparation: Silver nitrate is prepared industrially by reacting elemental silver with either dilute or concentrated nitric acid to give silver nitrate along with nitrogen oxides (NO or NO2) as byproduct.
3 Ag + 4 HNO3 (dilute) → 3 AgNO3 + 2 H2O + NO
Ag + 2 HNO3 (concentrated) → AgNO3 + H2O + NO2
Physical properties: Silver nitrate is found as a white odorless solid with a density of 4.35 g/mL, melting point of 210 °C and boiling point of 440 °C.
Chemical properties: Silver nitrate is water soluble and non-hygroscopic. Unlike many other silver salts, it is not sensitive to light. It is an oxidizing agent and is quite reactive as the nitrate ion can be easily replaced by other groups. Thus, it is a useful starting material for making many different silver compounds including silver halides, silver oxide, etc., as well as different metal nitrates such as copper nitrate. Silver nitrate is fairly stable to light and heat, but decomposes when heated to higher temperatures to give metallic silver along with toxic NO2 gas:
2 AgNO3 → 2 Ag + O2 + 2 NO2
Uses: Silver nitrate has several medical uses as it has antiseptic properties. Thus, it is used for treating some infections, warts, and ulcers and is also used as a cauterizing agent and a disinfectant. Its other applications include preparation of photographic films and explosives, manufacture of many silver compounds, analytical reagent, biological staining agent, and organic synthesis.
Health effects/safety hazards: Silver nitrate is toxic and corrosive and must be handled with care. It is used in very dilute solutions for medical applications. Skin or eye contact to small amounts of silver nitrate or its dilute solutions will cause greyish-black staining of the tissues (called argyria), while exposure to higher concentrations can cause burns.
|
Related Links: |
