Pyridine Formula
Pyridine is an organic base (imine) which has a structure related to benzene. It is mostly used as a basic dissolvent in organic reactions.
Formula and structure: The chemical formula of pyridine is C5H5N. Its structure is similar to a benzeno, with a CH- substituted by an N and its molar mass is 70.0999 g/mol. Pyridine is the main 6-atoms heterocycle and the N atoms is the responsible by the basic character of pyridine. Even in pyridine N replaces one of the CH- groups in benzene, the aromatic character of molecule is conserved, and however the pair of free electrons on the N does not participate in the resonance structure.
Occurrence: Pyridine is one of the heterocyclic compounds present in the mixtures of coal tar. It was for first time isolated from samples of coal tar in nineteenth century . Since that year, it was found that even when pyridine is not frequently found in nature, the pyridine rings occurs in a great variety of natural compounds as the alkaloids nicotine or actinidine.
Preparation: In nineteenth and the first part of twentieth century, commercial pyridine was obtained by distillation from crude coal tar. However, this process was inefficient due coal tar contains less than 1 % of pyridine. Today, most commercial pyridine is produced by synthetic processes. Some of these processes are:
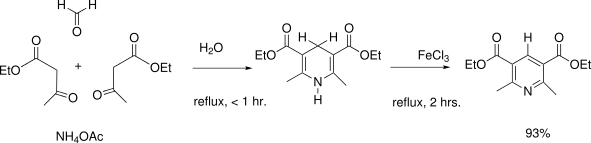
- Hantzsch pyridine synthesis uses a beta-ketoacid, an aldehyde and ammonia (or its salt) as nitrogen source.
- Bonnemann cyclization: its reaction is a trimerization of two molecules of acetylene and a nitrile molecule, in present of heat or light.
Physical properties: Pyridine is a colorless to light yellow liquid with nauseating fish-like smell. Its density is 0.978 g mL-1 and its boiling point is 115.2 ºC. It is miscible with water and other organic solvents.
Chemical properties: It is a weak base (pKa from the conjugated acid is 5.2) that is used as weakly basic catalyst in some organic reactions. Its less basic behavior let it be used as polar organic solvent for ionic reactions.
Uses: Pyridine is huge used as solvent in organic reactions despites its smell and toxicity due pyridine is cheap. Many industrial processes use pyridine as solvent due its capacity to act as solvent and weak basic. Moreover, pyridine can be used as precursor to obtain some interesting commercial compounds, for example the pesticides paraquat and diquat or anti-fungal substances.
Health effects/safety hazards: Pyridine is extremely toxic by ingestion and inhalation. Vapors are heavier than air. its combustion produces toxic oxides of nitrogen. Pyridine is highly flammable (its flash point is just 17 ºC). Pyridine also could have neurotoxic and genotoxic effects.
|
Related Links: |
