Propane Formula
Propane is a colorless gas, found in natural gas and used as combustible in houses and industries. The propane is used as mixtures of propane/ethane or propane/butane
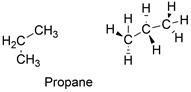
Formula and structure: The propane chemical formula is C3H8 and is extended formula is CH3CH2CH2. Its molar mass is 44.097 g mol-1. The molecule is formed by a chain of three carbon atoms which are bound to 3 or 2 hydrogen atoms in order to complete the 4 bonds required to complete the octet of Lewis structure. The carbon atoms are sp3 hybridized, thus the molecule has free rotation and a tetrahedral conformation. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Propane is found as a constituent of natural gas. It is also present in crude oil together with butane, methane, butylene, isobutylene and others where is extracted from distillation.
Preparation: Propane is producing by the distillation of crude oil or by extraction from natural gas. It is not largely synthesized by chemical industry and there is a few methods for producing it. In crude oil extraction, it is a sub-product in the process to obtain gasoline and other more commercial substances and it is removing to avoid pipelines problems.
Physical properties: Propane is a colorless and odorless gas, but it is shipped as liquefied compressed gas. These melting and boiling point are -187.6 ºC and -42.1 ºC, respectively. Its density is 2.009 g mL-1. Propane, in a pure form or when combined with methane, ethane or propane, can easily ignite forming vapors lighter than air. It is not soluble in water, but is slightly soluble in acetone, ethanol and more soluble in ether, chloroform and benzene.
Chemical properties: Propane is the third compound of the alkanes series, after methane and ethane. It has the same characteristics associated to alkanes, a low dipole moment that results in molecules, which can only interact through Van der Walls forces. This forces are originated by instantaneous dipoles in molecules as alkanes, which does not have a functional group to polarize the chain. Propane and butane are the last alkanes that are gases. Pentane and the rest of alkane with longer chain are liquids due the van der Waals forces are enough strong to originated effective and permanent intermolecular interactions.
Uses: Propane is largely used in houses as combustible. It is shipped as a liquefied gas in air comprised and join butane or ethane. It is used as biofuel in buses and taxis in many countries because it is cleaner than gasoline. Propane is also a gas used in the petroleum extraction through steam cracking.
Health effects / safety hazards: Propane is heavier than air, so that in high concentration it may cause asphyxiation by displaced the oxygen. Similar to other alkanes, propane is extremely flammable and can easily ignite. It may also explode by contact with fire, high temperature, strong oxidizing agents and halogens such as chlorine dioxide and nitrogen trifluoride.
|
Related Links: |
