Potassium tartrate Formula
Potassium tartrate is a salt of tartaric very used in food additive, especially as baking powder. It used to be confused with potassium bitartrate, which is extended used and known as cream of tartar.
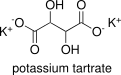
Formula and structure: Potassium tartrate, also known as dipotassium tartrate has a chemical formula K2(CH)2(OH)2(COO)2. Its molecular formula is K2C4H4O6 and its molar mass is 226.27 g mol-1. Potassium tartrate is a salt of tartaric acid, thus its structure keep the same functional groups: 2 hydroxyl groups (-OH) and two carboxyl group (-COO). In potassium tartrate, each carboxylic group binds to one potassium cation. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Potassium tartrate is formed as crystals in the wine casks during grape juice fermentation and can precipitate in wine bottle. This salt can also precipitate in the fresh grape juice bottles. The very relate salt, potassium bitartrate also precipitates in this conditions, but the bitartrate is present in a very large quantity when compared with potassium tartrate.
Preparation: Potassium tartrate is synthesized by the reaction of tartaric acid, Rochelle salt (potassium and sodium tartrate) and potassium sulfate. Rochelle salt is obtained when the bitartrate precipitated in winemaking reacts with caustic soda.
Physical properties: Potassium tartrate is odorless and slightly opaque crystalline powder. Its density is 1.984 g mL-1 and it is soluble in water but insoluble in alcohol.
Chemical properties: Potassium tartrate is salt from a dicarboxylic acid. In consequence, the negative charge is delocalized between the two oxygen atoms in each carboxylic group. Because the tartaric acid is dicarboxylic, it can be possible to form two different chemical species or anions when it is dissociated: potassium bitartrate (KC4H5O6) and tartrate (K2C4H4O6). Importantly, the potassium tartrate molecule has assymetric centers on carbon 2 and 3.
Uses: Potassium tartrate is extensively used in household applications, especially in culinary and cleaning of metals when combined with acids as lemon juice. In industry, the potassium tartrate and bitartrate are largely used as pH regulators.
Health effects/safety hazards: Potassium tartrate can be handled with normal care because there are few risks associated with its manipulation.
|
Related Links: |
Related Topics
Tartaric acid Formula
