Potassium Acetate Formula
Potassium acetate, also known as potassium salt E261, is a salt used in the pharmaceutical and food industries as an additive.
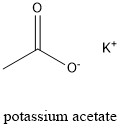
Formula and structure: Potassium acetate chemical formula is CH3COOK and it molar mass is 98.14 g mol-1. The molecule is formed by one potassium cation K+ and one acetate anion CH3COO-. The ions are bond by ionic bond, meanwhile the anion acetate is bond through covalent bonds having a resonance stabilization between both oxygen atoms. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Potassium acetate is not found in nature.
Preparation: Potassium acetate can be prepared through the reaction of acetic acid and potassium hydroxide or potassium carbonate, in an exothermic reaction:
CH3COOH + KOH → CH3COOK + H2O
2 CH3COOH + K2CO3 → 2 CH3COOK + CO2 + H2O
Physical properties: Potassium acetate is a white, deliquescent, crystalline powder. Its density is 1.8 g mL-1. The melting point of this salt is 292 °C, and at higher temperatures, it decomposes. Potassium acetate is soluble in water, ethanol, ammonia and methanol and insoluble in organic solvents.
Chemical properties: The anion acetate of the potassium acetate can be used in the neutralization of the metabolites through a replacement during the diabetic ketoacidosis: In this process, the level of potassium in blood can decrease, so the potassium acetate or other salts of potassium can be used to increase up to normal levels.
Uses: Potassium acetate is used during winter as a deicer to remove the ice formed. It is particularly used in airports due to it is less corrosive than other salts. Potassium acetate is also a extinguisher and a food additive. It is used as preservative and acidity regulator by the food industry. It is also used in the mummification of bodys.
Health effects / safety hazards: Potassium acetate can be an irritator of eyes and mucous. It is not flammable.
|
Related Links: |
