Magnesium Nitride Formula
Magnesium nitride is a inorganic compound used as catalyst in chemical synthesis.
Formula and structure: Magnesium nitride chemical formula is Mg3N2, and the molar mass is 100.95 g mol-1. The structure of magnesium nitride is formed by three magnesium cations Mg2+ and two nitrogen (III) anions N3-. Its chemical structure can be written as below, in the common representations used for organic molecules.
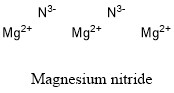
Occurrence: Magnesium nitride is found in nature as the mineral nitromagnesite.
Preparation: Magnesium nitride is prepared by the treatment of magnesium metal with nitrogen gas at high temperature:
3Mg + N2 → Mg3N2
Physical properties: Magnesium nitride is a pale yellow powder. Its density is 2.71 g mL-1. The melting point is 1500 °C and above this temperature decomposes to form nitrogen gas. It is freely soluble in water. Magnesium nitride is moderately soluble in ethanol, methanol and ammonia.
Chemical properties: Magnesium nitride is a ceramic with high hardness, high thermal conductivity and corrosion resistance properties that make this nitride very useful in the synthesis of new material. Sometimes, magnesium nitride is used in the production of other metallic nitrides and other times it is used to for special materials by forming alloys with other ceramics.
Uses: Magnesium nitride is used mostly in the production of pyrotechnics. It is also used as catalyst is some reaction to form other nitrides as the synthesis of synthetic diamond borazon (boron nitride), which is thermal resistant material and exceptional conductor. It can also be used to manufacture special glasses and ceramics. Magnesium nitride is also used to for magnesium hydroxide by adding water:
Mg3N2(s) + 6 H2O(l) → 3 Mg(OH)2(aq) + 2 NH3(g)
Health effects / safety hazards: Magnesium nitride is an irritant to the eyes, skin and respiratory tract. Particularly for eyes, it can cause severe damage in contact with. It is not flammable.
|
Related Links: |
