Lead (II) nitrate Formula
Lead (II) nitrate, also known as plumbous nitrate or lead dinitrate, is an inorganic salt used in the production of paints and coats since the Middle Age.
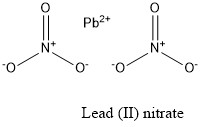
Formula and structure: The lead (II) nitrate chemical formula is Pb(NO3)2. The molar mass is 331.2 g/mol. The molecule is formed by one lead cation Pb2+ and two nitrate anions NO3-. The two anions are bound to the lead cation trough ionic bond. The geometry of the molecule is face-centered-cubic. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: It is not found in nature. Lead (II) nitrate is produced as described below.
Preparation: Lead (II) nitrate is produced from the dissolution of pure lead (metallic lead) in aqueous nitric acid, through the reaction:
Pb + 4 HNO3 → Pb(NO3)2 + 2 NO2 + 2 H2O
Alternatively, it can also be used lead (II) oxide:
PbO + 2 HNO3 → Pb(NO3)2 + H2O
Lead (II) nitrate is insoluble in nitric acid, thus the salt formed, precipitates and can be isolated by filtration.
Physical properties: Lead (II) nitrate is a white colorless crystal. The density of this salt is 4.53 g/mL (which is considered very heavy). Its melting point is 470 °C and above this temperature, it decomposes. Different from other lead (II) salts, lead (II) nitrate is very soluble in water, and the solubility increases with the temperature. It is poorly soluble in ethanol. It is insoluble in nitric acid.
Chemical properties: Lead (II) nitrate is soluble in water because differently to other lead (II) salts, the lead cation is bond to two nitrate anions. Nitrate anions are known for forming hydrogen bond with the water, thus helping in its solvation by water molecules.
Uses: Lead (II) nitrate was used as component of paints and coat. Nowadays, it has been replaced by titanium dioxide due to the toxicity of lead (II) nitrate. Today lead (II) nitrate is still used as component of some fireworks, stabilizer of some polymers and in component of photographic paper. It is used in the process in the gold cyanidation.
Health effects / safety hazards: Lead (II) nitrate is extremely toxic. It is toxic by ingestion and for humans and animals. It is classified as a probably carcinogenic to humans. It is also a teratogenic.
|
Related Links: |
