Hypochlorous Acid Formula - Hypochlorous Acid Uses, Properties, Structure and Formula
Hypochlorous acid is a weak acid which is typically formed when chlorine dissolves in water. It is referred to by several other names, such as: chloric acid, chloranol, hydrogen hypochlorite and chlorine hydroxide.
Formula and structure: The chemical formula of hypochlorous acid is HOCl. Its molecular formula is written as HClO and its molar mass is 52.46 g/mol. It is a simple molecule with the central oxygen connected to chlorine and hydrogen atoms through single bonds.
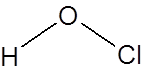
Occurrence: Hypochlorous acid is produced in the human body by the immune cells to fight infections, as it acts against a wide range of microorganisms.
Preparation: Addition of chlorine to water gives hypochlorous acid along with hydrochloric acid (HCl):
Cl2 + H2O → HOCl + HCl
The above reaction is in equilibrium, and it is not easy to isolate HOCl from this mixture. However, stable hypochlorous salts can be obtained by dissolving chlorine gas into sodium hydroxide solution, or other aqueous basic solutions.
HOCl can also be prepared by dissolving dichlorine monoxide in water.
Cl2O + H2O → 2 HOCl
Physical properties: Hypochlorous acid only exists as an aqueous solution. It is a colorless solution, and its exact physical properties are variable, since they depend on how concentrated the solution is. Anhydrous or dry hypochlorous acid is impossible to prepare since the molecule exists in equilibrium with its anhydride.
Chemical properties: HOCl is a strong oxidizer and can form explosive mixtures. In aqueous solutions, being a weak acid, it partially dissociates into the hypochlorite ion (OCl-) and H+.
HOCl reacts with bases to form salts called hypochlorites. For example, sodium hypochlorite (NaOCl), the active ingredient in bleach, is formed by reacting hypochlorous acid with sodium hydroxide.
HOCl + NaOH → NaOCl + H2O
Hypochlorous acid also readily reacts with a variety of organic molecules and biomolecules.
Uses: HOCl is a more powerful oxidizer than chlorine and a very effective sanitizing agent. It is used to make sodium hypochlorite (NaOCl) and calcium hypochlorite, (Ca(OCl)2), which are used in the making of bleaches, disinfectants and deodorants. Hypochlorous acid is the active sanitizer used in swimming pools. It is also used as a wound disinfecting agent, and a skin cleansing agent in cosmetics.
Health hazards/ health effects: Hypochlorous acid is not considered harmful, as it is produced in low concentrations in the human body, and has anti-microbial action.
|
Related Links: |
