Hydrogen peroxide Formula
Hydrogen peroxide is a chemical substance very used as bleach, oxidizing and anti-microbial agent.
Formula and structure: Hydrogen peroxide has the chemical formula H2O2. Its molecular formula is H2O2 and its molar mass is 34.0147 g mol-1. Hydrogen peroxide the simplest peroxide (compound with O-O bond) and its structure is H-O-O-H, thus hydrogen peroxide is called as "oxygenated water" because it is a water molecule with one more oxygen atom. The H2O2 is a nonplanar molecule with the O-O bond in a plane and the two hydrogen atoms are positioned in "V". This structure has a C2 symmetry axis and it is known as "open book geometry". Its chemical structure can be written as below.
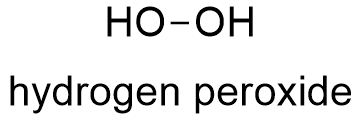
Occurrence: In nature, hydrogen peroxide is found as a defence agent produced by some species of beetles. Moreover, hydrogen peroxide has been found as a chemical compound of the immune system of zebrafish, where the quantity of peroxide is increased after some damage. Additionally, hydrogen peroxide has showed activity as a signaling molecule in some biological processes.
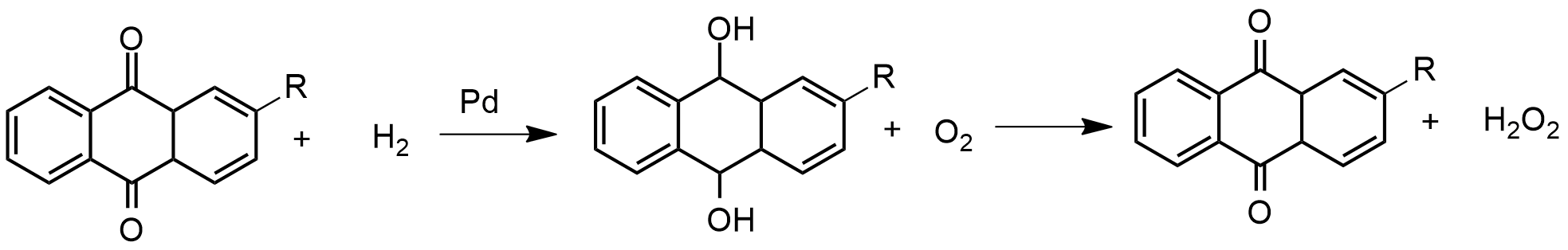
Preparation: Hydrogen peroxide is prepared in a reaction that starts with the reduction of a quinone to produce a hydroquinone. Generally, the process uses a palladium catalyst to perform the reduction, and then the hydroquinone suffers an autoxidation caused by bubbling air on the solution. The final products are the initial quinone and the hydrogen peroxide, which is removed by an extraction column, and then vacuum distillated. The general equation is:
Physical properties: hydrogen peroxide is a light blue to colorless and odorless liquid. Its density is 1.450 g mL-1. Its boiling (decomposition) point is 150.2 ºC. Hydrogen peroxide is miscible with water. Generally, it is commercialized as 30% (w/w) solution. The density of this solution is 1.11 g mL-1.
Chemical properties: Hydrogen peroxide is extensively use to obtain different products as other peroxides, epoxides, etc. Hydrogen peroxide is diluted to different concentration due concentrated H2O2 is highly reactive: it can explode if it is heated. Even though these risks, the diluted solutions are using in different reactions:
- Decomposition: when it is heated up to 150.2 ºC or in low pH to produce water and oxygen:
2H2O2 → 2H2O + O2
- Oxidation and Reduction: Hydrogen peroxide can be an oxidizing (one of the most potent) or reducing agent:
H2O2 + A → H2O + A-O (oxidation)
H2O2 + A → ZH2 + O2 (reduction)
Uses: The most known use of hydrogen peroxide is as disinfectant (solution 10-30% w/w) against bacteria and viruses in homes and hospitals. Other popular use is to bleach the hair. Hydrogen peroxide is used in different industrial process, especially as blenching and desinfectanting agent. In the production of paper is used to blench the wood pulp, similar usage has on the textile production. It is added to detergent due its blenching potential. Hydrogen peroxide is using in the wastewater treatment to oxidize phenols and other chemical compounds.
Health effects/safety hazards: Hydrogen peroxide diluted is safe and approved by the Food and Drug Administration as antimicrobial agent. However, when concentrate; hydrogen peroxide can irritate the skin, eyes and other mucous membranes. It explodes violently when heated and the contact with combustible materials or metals such as copper, iron, nickel or zinc, may result in ignition.
|
Related Links: |
Related Topics
Atoms
Compounds Examples
Theoretical Yield Formula
Sodium cyanide Formula - Sodium cyanide Uses, Properties ...
Potassium cyanide Formula - Uses, Properties, Structure and Formula
