Hydrogen Formula
Hydrogen, also known as H2, is a gas that is present in traces in the atmosphere and can be used in batteries.
Formula and structure: The hydrogen chemical formula is H2. The molar mass depends on the hydrogen isotope you are considering; the hydrogen (formed by a two nuclei with one proton each one) has a molar mass of 2,00 g/mol; the deuterium (formed by a two nuclei with one proton and one neutron each one) has a mass of 4,00 g/mol and the tritium (formed by a two nuclei with one proton and two neutron each one) has a mass of 6,00 g/mL. The molecule is formed by two hydrogen atoms joined by one ionic bond. Its chemical structure can be written as below, in the common representations used for organic molecules.
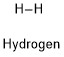
Occurrence: Hydrogen is the most abundant gas in the universe, however in the Earth, is found in traces in the atmosphere and in some planets and stars as Jupiter and the Sun. It can also be produced by some micro-organisms.
Preparation: Hydrogen is produced by some methods:
It can be produced by the electrolysis of water resulting in molecular oxygen and hydrogen:
2H2O → 2H2 + O2
When produced by micro-organisms, the H2 is biosynthesized by the action of enzymes called hydrogenases.
Physical properties: Hydrogen is a colorless gas. The density of this gas is 0.089 g/mL. Its melting point is -259.16 °C and its boiling point is -252 °C.
Chemical properties: Hydrogen gas is made of two molecules of elemental hydrogen H. In general, it is considered very reactive because the mixtures with air can be explosive, but only at temperature up to 500 °C. At room temperature, it is not reactive. Hydrogen gas can react with other elemental compounds as halogens to forms hydrides and acids:
Cl2 + H2 → 2HCl
S2 + 2H2 → 2H2S
Uses: Hydrogen can be used as a reactant in the production of ammonia and some acids as HCl and H2S. It is also used in the production of petroleum oil. Hydrogen can also be used to produce batteries or to produce energy through the nuclear fusion power plants.
Health effects / safety hazards: Hydrogen is harmful to human health. It can explode when mixture with air and at high temperatures. It does not react with other compounds.
|
Related Links: |
