Glycerol Formula
Glycerol, also known as glycerin, is a triol compound that essential part of lipids from plants and animals. It is used in dermatological treatments. More recently, it has largely used as biofuel.
Formula and structure: The glycerol chemical formula is C3H8O3 and its extended formula is CH2OH-CHOH-CH 2OH. The IUPAC name for glycerol is 1, 2, 3- Trihydroxypropane or 1, 2, 3- Propanetriol. Its molar mass is 92.09 g mol-1. The molecule is a 3 carbon chain, which has 3 hydrogen substituted by 3 hydroxyl group (-OH). All carbon atoms have sp3 conformation, thus the molecule has free rotation in all bonds. The glycerol molecula is also prochiral. Its chemical structure can be written as below, in the common representations used for organic molecules.
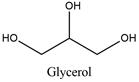
Occurrence: Glycerol is a triglyceride present in many plants such as soy bean or palm and animals, where is produced from saponification or hydrolysis of triglycerides.
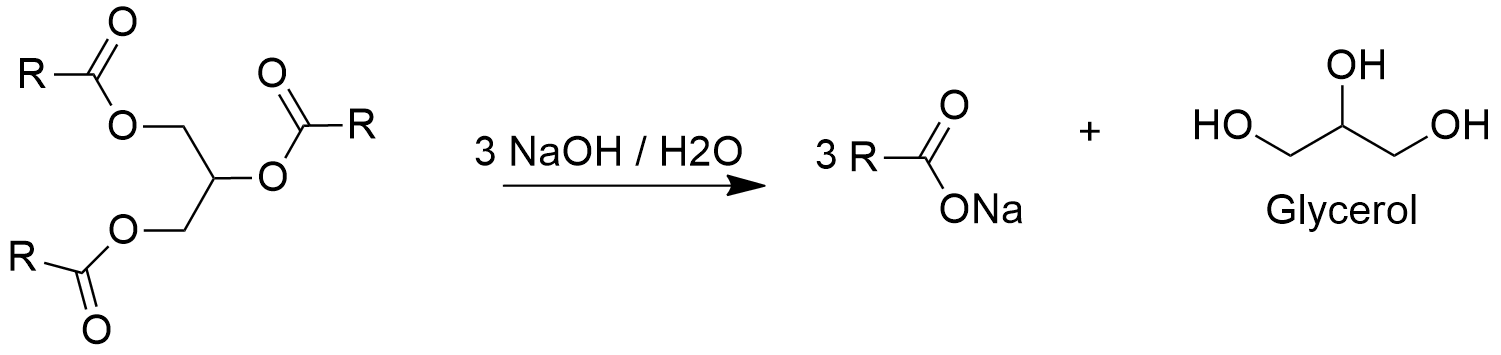
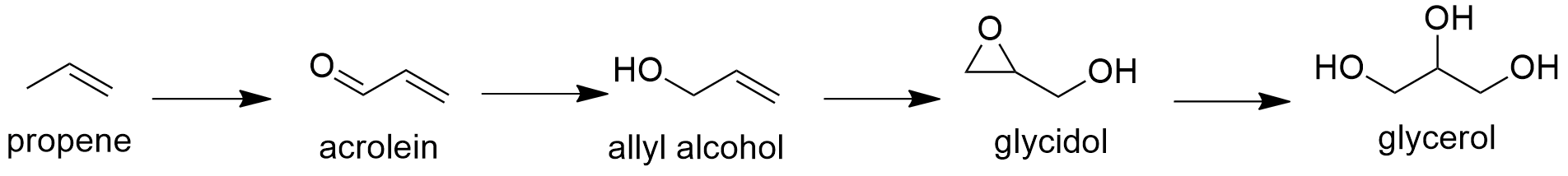
Preparation: Glycerol is mostly extracted from plants and animal tissues. Particularly, its production has increased in the last years due its capacity to be used as biofuel (combustible alternative to fossil fuel). Additionally, there are some organic synthetic procedures to obtain glycerol; for example, the production of glycerol from acrolein that consist in a first oxidation of propene to acrolein, followed by a reduction to yield allyl alcohol. Then, this alcohol reacts in presence of hydrogen peroxide to form glycidol through a epoxidation. The last step consists in the hydrolysis of glycidol to glycerol.
Physical properties: Glycerol is a clear, colorless and very viscous liquid, similar to a syrup. The melting and sublimation points are -20 ºC and -290 ºC, respectively. Its density is 1.250 g mL-1. It is highly hygroscopic. It is soluble in water. Glycerol is combustible but cannot ignite easily.
Chemical properties: Glycerol is a triol, which means glycerol has 3 hydroxyl groups. The presence of these group results in a high solubility in water and other polar protic solvents due its capacity to form hydrogen bonds. The presence of hydroxyl groups also are responsible of the hygroscopic character. Glycerol presents in lipid tissues is a precursor fro the biosynthesis of triglycerides mediated by various enzymes such as glycerol kinase.
Uses: Glycerol is used in pharmaceutical and personal care formulations as emollient and hydrant, for example: the glycerin soaps or laatives. It is widely used in molecular biology laboratories in the preparation of polyacrylamide electrophoresis gel for proteins and also as preservation crio-agents for storage biosamples. Glycerol is also used as sweetener and preserver in foods, it is particular used as an alternative to sugar because it contains lesser calories. Additionally, glycerol is used as antifreezer for cars and to produce the explosive component trinitroglycerin.
Health effects / safety hazards: Glycerol can cause serious eye damage. It is also highly combustible and there is serious risk of explosion when it is heated. Moreover, glycerol is incompatible with hydrogen peroxide, potassium permanganate, sulfuric acid, nitric acid, nitrobenzene. It can also explode in presence of potassium peroxide, acetic acid and hydrochloric acid.
|
Related Links: |
