Ethylene Formula
Ethylene, also known as ethene, is a chemical compound largely used in chemical industry, particularly in the production of polymers such as polyethylene.
Formula and structure: The ethylene chemical formula is C2H4 and its extended formula is CH2=CH2. Its molar mass is 28.04 g mol-1. The molecule is the simplest alkene, a functional group characterized by having double bonds. The acetylene molecule is planar, with 120 ºC separating the simple bonds; this geometry resulting from the carbon atoms hybridized sp2. Both carbons has 3 sp2 orbitals: two of them bound to two hydrogen atoms and the third for the carbons simple bond. The double bond, also called Π bond, is formed between the two orbitals P without hybridization, that are orthogonal to the planar system. Its chemical structure can be written as below, in the common representations used for organic molecules.
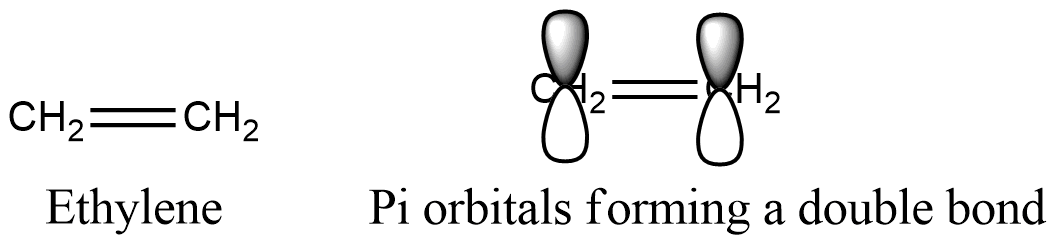
Occurrence: Ethylene is found in some hormones from plants. It is thought, ethylene is a regulator of many plants processes, such as: maturation of fruits or opening of flowers. Consequently, ethylene is essential for plants development.
Preparation: Ethylene commercially available is mostly prepared from organic synthesis. It is produced from ethanol and sulfuric acid through a dehydration reaction:
CH3CH2OH + H2SO4 → CH2=CH2 + H2O + SO2
Ethylene is also extracted from plants where is found in roots, flowers, fruits, leaves, stems and seeds. It is biosynthesized from the amino acid methionine.
Physical properties: Ethylene is a colorless gas with sweet odor and taste. The melting and boiling points are -169.2 ºC and -103.7 ºC, respectively. Its density is 1.178 g mL-1. It may easily ignite forming vapors lighter than air. Ethylene is soluble in acetone and benzene. It is no soluble in water.
Chemical properties: The ethylene is a molecule very reactive due the electrons from Π orbitals. This type of bonds is very rich in electron density, so that the molecule can attack other chemical compound deficient in electrons (known as electrophiles). In consequence, ethylene can suffer a huge variety of reactions.
Uses: ethylene is used in chemical industry in many reactions, such as: polymerization, oxidation, halogenation, alkylation and hydration. However, the most extended use is in the production of the most used plastic, called polyethylene (polymerization) and ethylene oxide (oxidation).
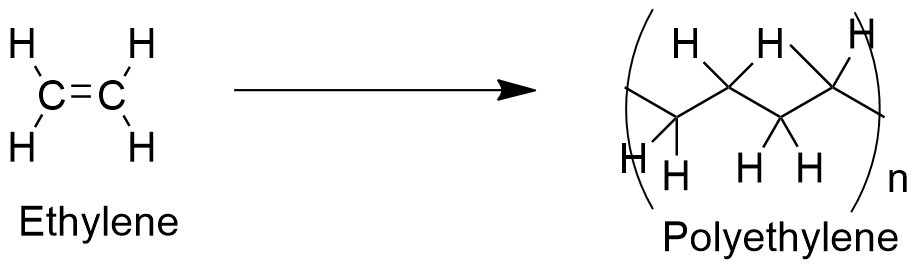
Ethylene is also used as intermediates in halogenation to produce ethylene dichloride, ethylchloride and ethylene dibromide. Moreover, ethylene is used as precursor to obtain ethanol.
Health effects / safety hazards: Ethylene is highly toxic and it may cause asphysxia by displaced of air. It is also a confirmed carcinogenic compound and the long-term exposition must be avoid. Ethylene is also combustible and can ignite by heat or fire exposition. It decomposes when heated yielding acrid smoke and irritating fumes.
|
Related Links: |
