Ethyl alcohol Formula
Ethyl alcohol, also known as ethanol, is an alcohol found in alcoholic beverage and which is produced by yeasts. It is also used as a solvent and as a raw material in chemical industry.
Formula and structure: Ethyl alcohol chemical formula is C2H5OH and its extended formula is CH3CH2OH. It is also written as EtOH and the IUPAC name is ethanol. Its molar mass is 46.06 g mol-1. The molecule is formed by a two carbon chain (ethane), in which a H has been substituted by a hydroxyl group (-OH). It is the second more simplest alcohol. All the carbon and the oxygen atoms are sp3 allowing the free rotation of molecule bounds. Its chemical structure can be written as below, in the common representations used for organic molecules.
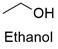
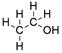
Occurrence: Ethanol can widely be found in nature due it is part of the metabolic process of yeast such as Saccharomyces cerevisiae and it is also present in the mature fruit. It is also produced by some plants through the anerobiosis and it has been found in outer space too.
Preparation: Ethanol can be produced by yeast using sugar fermentation or through organic synthesis. The organic synthesis is made through the hydration of ethylene obtained in the petrochemical industry and using sulfuric or phosphoric acid as catalyst at 250-300 ºC:
CH2=CH2 + H2O → CH3CH2OH
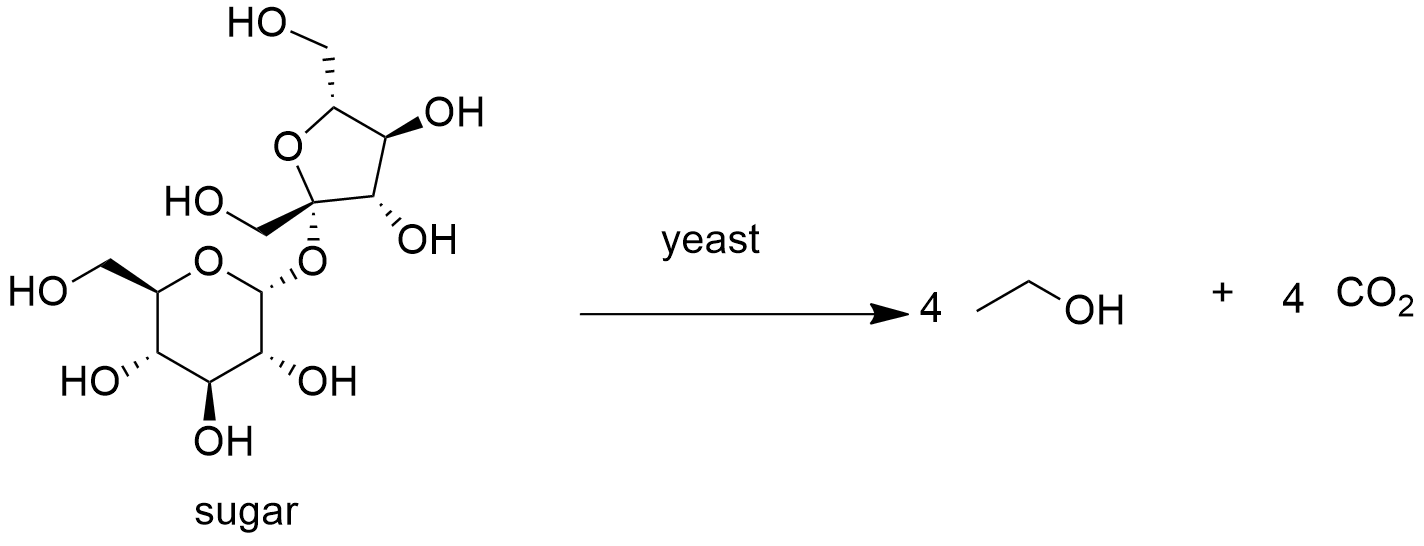
Ethanol from fermentation of sugars is the main process to produce alcoholic beverages and bio- combustibles. It is largely used in countries as Brazil, where it is used yeast for the biosynthesis of ethanol from sugar cane:
Physical properties: Ethyl alcohol is a colorless with slightly odor liquid. Its melting and melting point are -114 ºC and 78 ºC, respectively. It is a volatile liquid and its density is 0.789 g mL-1. Ethyl alcohol is also flammable and it produces a blue flame without smoke. It is miscible with water and in most of the organic solvents such as acetic acid, acetone, benzene, carbon tetrachloride, chloroform and ether. Interesting, ethanol is also miscible in aliphatic solvents such as pentane and hexane, but its solubility depends on the temperature.
Chemical properties: Ethanol is the best known representative of alcohol. In this molecule, the hydroxyl group is in a terminal carbon, resulting in a high polarization of molecule. In consequence, ethanol can form strong interactions, such as hydrogen bonds and dipole-dipole interaction. In water, ethanol is miscible and the interactions between the two liquids is so high, that results in a mixture known as azeotrope, with characteristics different to the two components.
Uses: Ethanol is used by the pharmaceutical industry as an antiseptic and it is also a component of many medicines, cosmetics and health care products. It is also one of the most used solvent in chemical and biotechnological industries. Ethanol is also a raw material and intermediates in reaction of oxidation halogenation and dehydration. More recently, ethanol has been used as a biofuel in alternative to gasoline.
Health effects / safety hazards: Ethanol is toxic when ingested in large quantities. It acts in the central nervous system as depressant and diuretic. It is also irritating for eyes, through and nose. It is highly flammable and reacts violently with peroxides, acetyl chloride and acetyl bromide. When in contact with some platinum catalysts may ignite.
|
Related Links: |
