DABCO (Triethylenediamine) Formula
Triethylenediamine, more known as DABCO or 1,4-Diazobicyclo[2.2.2]octane, is an organic compound better used as catalyst and reagent in organic synthesis.
Formula and structure: The chemical formula of DABCO is C6H12N2. It molecular mass is 112.76 g mol-1.The structure is a bridge bicycle (molecule formed by two joined rings which share three or more atoms) which one nitrogen atom in each bridgehead. The chemical structures can be written as below, in the common representations used for organic molecules.
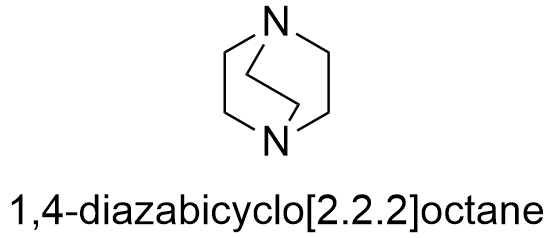
Occurrence: DABCO is not found in nature.
Preparation: DABCO can be produced through the reaction of ethylenediamine or ethanolamine with different catalysts. It can also be produced from diethanolamine or diethylenetriamine.
3 H2NCH2CH2OH → N(CH2CH2)3N + NH3 + 3 H2O
Physical properties: DABCO is a white crystalline powder. Its melting point is 156-160 °C and its boiling point is 174 °C. It is soluble in water and also soluble in organic solvent as ethyl acetate, ethanol, methanol, benzene and acetone. DABCO is hygroscopic.
Chemical properties: DABCO is highly nucleophilic amine due to the nitrogen atoms located on the bridgeheads have two pair of electron that can react with deficient species and additional, they are unhindered.
Uses: DABCO can be used as a catalyst and also as reagents in organic synthesis reactions. One of the most common or extended uses are for Bayllis-Hillman reaction between aldehydes and unsaturated ketones. It is also used as reagent for polymerization in the production of polyurethane.
Health effects/safety hazards: DABCO is flammable. It is toxic to the aquatic life and can cause several damages to eyes and skin. It can also cause respiratory irritation.
|
Related Links: |
