Copper (II) Oxide Formula
Copper (II) oxide, also known as cupric oxide or the mineral tenorite, is an inorganic compound used as precursor or raw material in the production of chemical products that contain copper.
Formula and structure: Copper (II) sulfate chemical formula is CuO and its molar mass is 79.55 g mol-1. The molecule is formed by one Cu2+ cation bond to a oxygen anion O2-. The cystral structure is a monoclinic crystal system with a copper atom coordinated by 4 oxygen ions. Its chemical structure can be written as below, in the common representations used for organic molecules.
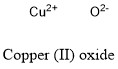
Occurrence: Copper (II) oxide is found in nature as being part of the mineral tenorite and it is extracted with the traditional mining techniques.
Preparation: Although copper (II) oxide can be found in nature, it is not extracted in a large scale, so that industrial synthesis of this compound is required. The CuO is prepared through the reaction of copper metal and oxygen gas at high temperature >500 °C:
2 Cu + O2 → 2 CuO
Physical properties: Copper (II) oxide is a black to dark brown powder. The oxide density is 6.31 g mL-1. The melting point is 1326 °C , while the boiling point is 2000 °C. It is not soluble in water, although it is soluble in ammonium chloride and potassium cyanide solutions. It is also insoluble in ethanol.
Chemical properties: Due to the capacity of copper (II) oxide to act as an oxidizing agent, it can be used in the treatment of hazardous materials as the very toxic cyanide or halogenated hydrocarbons. The residues of the reactions, that include copper metal, can be disposing in a safe way. The main reactions are:
C6H5OH + 14CuO → 6CO2 + 3H2O + 14Cu
C6Cl5OH + 2H2O + 9CuO → 6CO2 + 5HCl + 9Cu
Uses: Cupric oxide is used in the production of copper salts as the copper sulfates or copper nitrates. It is used in the production of dyes, pigments and paints due to the characteristic blue and green color associated with the Cu2+ cation. It is also used in some batteries as the lithium-copper batteries.
Health effects / safety hazards: Copper (II) oxide is not toxic to humans and animals in low concentration. In some cases, it can be found in water. However in high concentration, it can be toxic for the aquatic life. It can explode when heated with with powdered aluminum, anilinium perchlorate, hydrogen or magnesium.
|
Related Links: |
