Cobalt (II) chloride Formula
Cobalt (II) chloride, also known as cobaltous chloride or muriate of cobalt, is an inorganic salt, mostly used in organic synthesis methodologies as a source of cobalt.
Formula and structure: The cobalt (II) chloride chemical formula is CoCl2. This molecule present different level of hydratation: anhydrous (CoCl2), dihydrate (CoCl2.2H2O) and hexahydrate (CoCl2.6H2O) which have molar mass of 129.839 g mol -1, 165.87 g mol-1 and 297.93 g mol-1. In general, the molecule is formed by the cobalt cation Co+2 and chloride anion Cl- and their structures are hexahonal for the anhydrous form and monoclinic and octahedral for the dihydrate and hexahydrate complex. Its chemical structure can be written as below, in the common representations used for organic molecules.
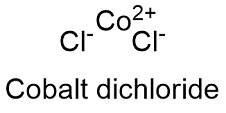
Occurrence: Cobalt dichloride can be found in nature, especially in rocks and minerals but also can be found in soil.
Preparation: Cobalt dichloride is obtained from the cobalt dihydroxide or cobalt (II) carbonate, which reacts with hydrochloric acid in water to form a hexahydrate salt of cobalt dichloride. After heated, this salt is dehydrated and cobalt dichloride can be recovery.
CoCO3 + 2 HCl + 5 H2O → Co(H2O)6Cl2 + CO2 → CoCl2 (after heated)
Physical properties: Cobalt dichloride anhydrous is blue crystalline powder. It is hygroscopic and its density is 3.35 g mL-1. Cobalt dichloride melting point is 724 ºC and its boiling point is 1049 ºC. It is soluble in water and ethanol, pyridine, acetone, ether and methanol.
Chemical properties: Cobalt dichloride can act a weakly acidic salt, thus it can be used to react and neutralized weak bases. This reaction generally is exothermic, so that generate heat and should be made carefully. Cobalt dichloride is molecule relatively poor to react in REDOX reactions due does not have enough electron to lose or gain.
Uses: Cobalt dichloride is used by the chemical industry to generate some precursors to produce other cobalt compounds. For example: with amines or ammonia, cobalt dichloride can react to form a lot of cobalt (II) complexes. It is also used as a component of materials with thermoelectric, magnetic and oxidation-resistance properties. Cobalt (II) dichloride or other Cobalt (II) salts are used as indicator for water in desiccants.
Health effects / safety hazards: Cobalt (II) chloride is a substance classified as suspected carcinogen, so that it must be manipulated correctly. It also causes respiratory disease and coughing. Ingestion causes diarrhea and it is also an irritant to eyes. It is not flammable, however, when heated toxic and combustible cobalt oxides fumes are emitted.
|
Related Links: |
