Carbonic acid Formula - Carbonic Acid Uses, Properties, Structure and Formula
Carbonic acid is a weak inorganic acid, which is also considered a solution of carbon dioxide in water. It only exists as a solution, and is also called acid of air, aerial acid, carbon dioxide solution, or dihydrogen carbonate. It is best known as a component of most aerated drinks, such as sodas and soft drinks.
Formula and structure: The chemical formula of carbonic acid is H2CO3. Its molecular formula is CH2O3, and its molar mass is 62.03 g/mol. The chemical structure of carbonic acid is shown below, and it consists of a carboxyl group, and two hydroxyl groups. It is a diprotic acid that can release two protons, but is only weakly acidic due to the strong O-H bonds.
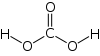
Occurrence: In the human body, CO2 present in the blood combines with water to form carbonic acid, which is then exhaled as a gas by the lungs. It is also found in rocks and caves where it can dissolve limestones. H2CO3 can also be found in coal, meteors, volcanoes, acid rain, ground water, oceans, and plants.
Preparation: Carbonic acid is formed when carbon dioxide is dissolved in water and can only exist in a solution. This reaction is typically in equilibrium, meaning that carbonic acid partially and reversibly forms carbon dioxide and water.
CO2 + H2O ⇌ H2CO3
Industrially, carbonic acid is obtained as the by-product of other processes such as fermentation, fossil fuel burning, etc.
Physical properties: Carbonic acid only exists as a solution, with a density of 1.668 g/mol. It is insoluble in water.
Chemical properties: Carbonic acid is a weak and unstable acid, which partially dissociates in water into hydrogen ions (H+) and bicarbonate ions (HCO3-). Being a diprotic acid, it can form two kinds of salts, carbonates and bicarbonates. Addition of base to an excess of carbonic acid gives bicarbonate salts, while addition of excess base to carbonic acid gives carbonate salts.
Uses: Carbonic acid is widely used in the preparation of bubbly drinks such as sodas, soft drinks, sparkling wines, and other aerated beverages. Carbonic acid is also used in many other fields, such as pharmaceuticals, cosmetics, fertilizers, food processing, anesthetics, etc.
Health hazards/ health effects: Carbonic acid is not considered toxic or hazardous, and is present in the human body. However, its exposure at high concentrations can irritate the eyes and respiratory tract.
|
Related Links: |
