Calcium chloride Formula - Calcium chloride Uses, Properties, Structure and Formula
Calcium chloride is an important calcium salt that has many household and industrial applications.
Formula and structure: The chemical formula of calcium chloride is CaCl2, and its molar mass is 110.983 g/mol. It is an ionic compound consisting of the calcium cation (Ca2+) and two chlorine anions (Cl-). The bivalent calcium metal forms an ionic bond with two chlorine atoms, as shown below.
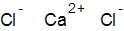
Occurrence: Calcium chloride occurs in nature in its hydrated forms as the rare minerals sinjarite (dihydrate) and antarcticite (hexahydrate). It is also found in large amounts in natural brine from salt lakes and salt deposits.
Preparation: Calcium chloride is mainly produced by reacting limestone (CaCO3) with hydrochloric acid (HCl).
CaCO3 + 2 HCl → CaCl2 + CO2 + H2O
It is also produced as a major by-product during manufacture of soda ash (Na2CO3) by the Solvay process, in which limestone is reacted with NaCl solution.
Physical properties: Calcium chloride is found as an odorless white powder, granules or flakes. It has a density of 2.15 g/mL, melting point of 782 °C and a high boiling point over 1600 °C.
Chemical properties: CaCl2 is highly water soluble, hygroscopic (absorbs moisture from air) and deliquescent (absorbs enough water to turn into liquid). Calcium chloride dissolves in water in a very exothermic manner (releasing a large amount of heat). Calcium chloride fully dissociates in water to give calcium cations and chloride anions. In aqueous solutions, the calcium ions can displace other ions in exchange reactions, such as the conversion of potassium phosphate into calcium phosphate:
3 CaCl2 + 2 K3PO4 → Ca3(PO4)2 + 6 KCl
Uses: Calcium chloride has several similar uses as sodium chloride, and it is used as a food additive, food preservative, for de-icing roads in winter, and as brine in refrigeration plants. It is also used as a swimming pool chemical, in water treatment plants, and for desiccating purposes. It also has applications in metallurgy, oil-well drilling, and rubber, paper, dye and paint industries.
Health effects/safety hazards: Calcium chloride is not toxic in normal amounts and is used in foods. However, its main hazard is due to its desiccating (drying) property and exothermic reaction when it dissolves in water. Thus, when the solid comes into contact with wet skin or eyes, it can cause severe irritation and mild burns. Swallowing large amounts can result in nausea, vomiting and abdominal pain.
|
Related Links: |
