Butane Chemical Formula
Butane, also known as n-butane or refrigerant 3-11-0, is a largely used organic compound that is used as gasoline blender and organic solvent.
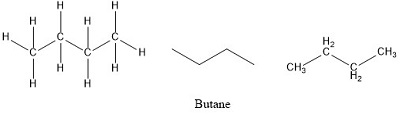
Formula and structure: The butane chemical formula is C4H10. The molar mass is 58.12 g/mol. The molecule is the fourth more simple alkane, formed by 4 carbon atom and 10 hydrogen atoms, bound by simple bonds CH3CH2CH2CH3. The geometry of the molecule is tetrahedral with all the carbon atom being sp3 and having bonds of 109° of angle. Butane also has a isomer, called methyl butane with the formula CH3(CH)CH3CH4. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Butane is found as occurring naturally in the same well that petroleum. It is found in the form of natural gas. By extracting petroleum, it is started a process in which all the fractions and different products contained in the petroleum are separated into pure compounds. One of the product of this separation is the butane.
Preparation: Butane is not synthesized; it is just extracted from natural sources.
Physical properties: Butane is a colorless gas. The density of this gas is 2.48 g/mL. Its melting point is -140 °C and the boiling point is -1 °C. Butane is soluble in water and in organic solvents. It is also used as a organic solvent at low temperatures.
Chemical properties: Butane can be used in a large amount of reactions. One of them, it is its combustion, forming water and carbon dioxide. This reaction is not exclusive of butane, and instead it is very known for all the alkanes.
2 C4H10 + 13 O2 → 8 CO2 + 10 H2O
If oxygen is limited, it will be formed CO instead of CO2.
Butane can also be used in a very well know industrial reaction to produce maleic anhydride:
2 C4H10+ 7 O2 → 2 C2H 2(CO)2O + 8 H2O
Uses: Butane is used as a component of gasoline and fuel gas. At low temperatures, butane can be used in the extraction of fragrances and in the production of some chemical compounds as butadiene. In chemical industry, it is used in the production of synthetic rubber and other compounds produced from petroleum as plastic and sprays.
Health effects / safety hazards: Butane can cause damage for health. Butane inhalation can cause euphoria, asphyxia and in death. Butane is extremely flammable.
|
Related Links: |
