Aluminum Sulfate Formula
Aluminium sulfate is an inorganic salt, also known as cake alum or alunogenite, that is used as immune adjuvant activity.
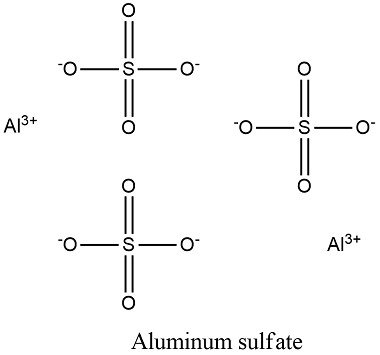
Formula and structure: the aluminum sulfate chemical formula is Al2(SO4)3 and the molar mass of the anhydrous molecule is 342.15 g/mol, meanwhile the octahydrate salt has a molar mass of 666.44 g/mol. The salt forms monoclinic crystal, with two central aluminium atoms, surrounded by three sulfate ions. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Aluminium sulfate can be found in nature in some minerals and ore of bauxite, clays and cryolite. In some cases, such as the bauxite, the material needs to be calcined to get the aluminium sulfate.
Preparation: The preparation of aluminium sulfate is made using the reaction of aluminium hydroxide Al(OH)3 with sulfuric acid:
2 Al(OH)3 + 3 H2SO4 → Al2(SO4)3 + 6H2O
Physical properties: Aluminium sulfate is a White, hygroscopic, crystalline solid. The density of the anhydrous salt is 2.672 g/mL and in the case of the octadecahydrate it is 1.62 g/mL. Its melting points are 770 ºC (anhydrous salt) and 86.5 ºC(octadecahydrate salt). It is highly soluble in hot water and less soluble in cold water.
Chemical properties: Aluminium sulfate has interesting chemical properties that are used in immunology. This salt is able to adsorbs and precipitate antigens in solution. This antigen precipitates help to increase the vaccine immunogenicity because it is easy to release the antigens in these conditions.
Uses: Aluminium sulfate is used for precipitating aluminium oxide in alkali solutions and this precipitate is useful in some chemical processes and also in water purifiation. This properties is also useful for reducing the pH of soil where a small quantity of aluminium sulfate reacts forming Al(OH)3 and H2SO4.
Health effects/safety hazards: Aluminium sulfate is corrosive to metals and can cause serious eye irriation. It is not flammable.
|
Related Links: |
