Acetaminophen Formula
Acetaminophen, also known as paracetamol or APAP, is a medication used as anti-inflammatory and anti- pyretic. It is on the World Health Organization List of Essential Medicines needed in any health system.
Formula and structure: The acetaminophen has the IUPAC name N-(4-hydroxyphenyl)acetamide and its chemical formula us C8H9NO2 and its extended formula is HOC6H4NHCOCH3.Its molar mass is 151.165 g mol-1. The molecule is formed by an aromatic phenyl ring, which has two substituents in position -para (1,4). The first substituent is an amide group (acetamide) and the second is a hydroxy group (-OH). The molecule is planar with 7 carbon atoms with sp2 hybridization. Its chemical structure can be written as below, in the common representations used for organic molecules.
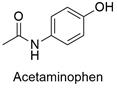
Occurrence: Acetaminophen is not found in nature. Similar to aspirin, it was synthesized by the first time in 1877 by Harmon Northrop Morse, however it was not used as anti-pyretic until 1893, after researchers from Germany found the paracetamol in the urine of patients which were being treated with phenacetin. Today, it is known, the paracetamol is part of the metabolism of degradation of phenacetin.
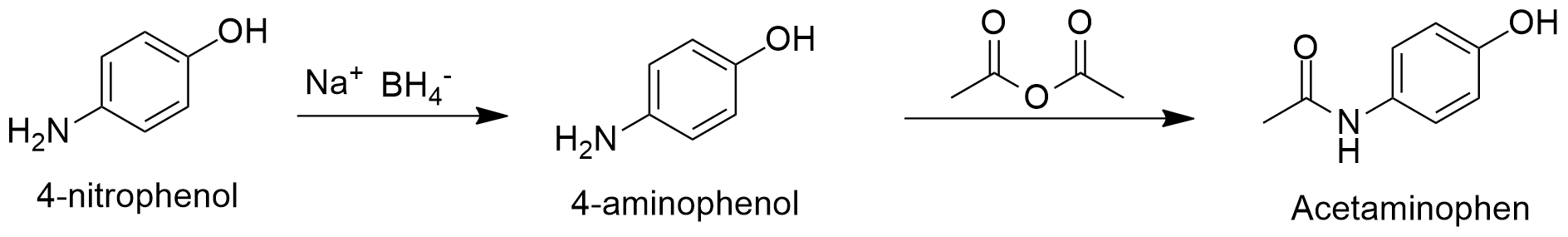
Preparation: Acetaminophen can be synthesized by the acetylation of 4-aminophenol when treated with acetic acid and acetic anhydride at high temperatures. It can also be prepared by the treatment of acetic anhydride in pyridine or with acetyl chloride and pyridine in tolueno. All reactions are performed at >70 ºC. The first step of this process is the obtention of 4-aminophenol through the reduction of 4-nitrophenol in presence of sodium borohydride.
Physical properties: Acetaminophen is a colorless to white crystalline solid. It is odorless and it has a bitter taste. Acetaminophen density is 1.293 g mL-1. Its melting and boiling point are 168 ºC and 420 ºC, respectively. It is soluble in boiling water, ethanol, methanol, dimethylformamide, ethylene dichloride, ethyl ether and acetone. It is insoluble in ether, cold water and benzene.
Chemical properties: Acetaminophen mechanism of action is thought as similar to aspirin, through the inhibition of enzyme cyclooxygenase, causing the suppression of Prostaglandin production. Moreover, it is also inhibit the nitric oxide route to increase the pain threshold. The paracetamol molecule is extremely reactive in the body due its chemical structure: it has two activator substituents (-OH and -NCOR) which results in very reactive molecule toward aromatic substitution.
Uses: Acetaminophen is largely used to reduce fever and pain in people, including children. Moreover, it is also used in the treatment of arthritis. It is used as raw material and intermediate in the production of other medicines. Acetaminophen is mostly used through the oral consumption.
Health effects / safety hazards: At the same way that other medicines, acetaminophen long term consumption can be dangerous to health, especially to liver. It does not cause gastric irritation as the aspirin. It may cause irritation to eyes.
|
Related Links: |
